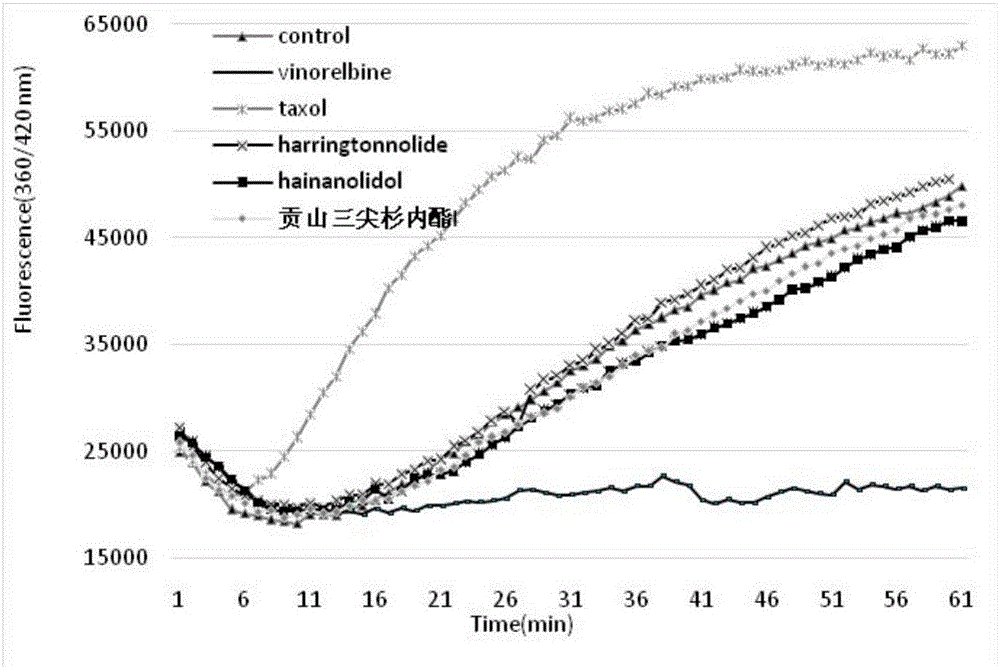
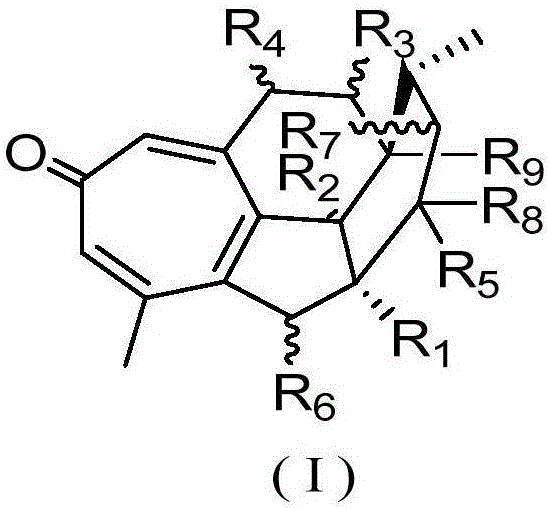
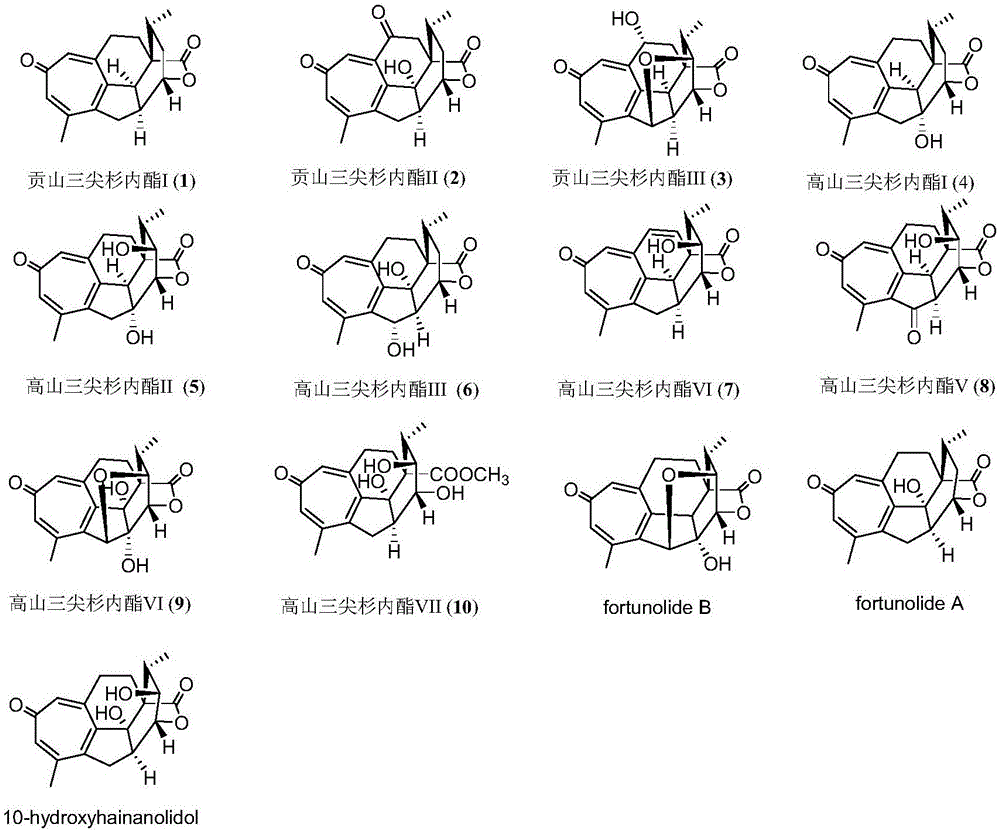
Cephalotaxus fortunei norditerpenoid compounds as well as pharmaceutical composition and application to pharmacy thereof
A technology of cloverleaf and compounds, applied in the field of medicine, can solve the problems of compound activity mining, less research on mechanism of action, and less quantity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0053] Extraction and Separation
[0054] Dried alpine clover (39kg) and Gongshan clover branches and leaves (19kg) were crushed and soaked in methanol 3 times for 2 days each time, the extracts were combined, the solvent was distilled off under reduced pressure, and the extract was diluted with water. Add 1% (v / v) hydrochloric acid aqueous solution to adjust the pH to 2–3 and stir, extract with petroleum ether to remove impurities such as chlorophyll and fatty acids, and extract the aqueous layer with ethyl acetate to obtain crude extract A 1 (510g) and B 1 (292g), the pH of the aqueous layer was adjusted to pH 7–8 with 10% ammonia (v / v) solution, and then extracted with ethyl acetate to obtain the crude extract A 2 (198g) and B 2 (39g).
[0055] Take the crude extract of Alpine cloverleaf A 1 (252g) was mixed with 250g normal phase silica gel, 3kg silica gel column chromatography, chloroform-methanol (1:0–1:1, v / v) gradient elution, detected by thin layer chromatography,...
preparation Embodiment 2
[0062] Chemical transformation:
[0063] Under alkaline conditions, the lactone ring can be opened. For example: adding the cephalus triterpene compound containing the lactone ring to the sodium methoxide / methanol solution, stirring at room temperature, filtering, and drying to prepare the lactone ring. Similar compounds.
[0064] Obtain unsubstituted diterpenes through dehydroxylation reactions such as: adding pyridine and phenyl thiochloroformate ( PhOCSCl) after 7–14 hours of reaction, add tri-n-butyl tin hydrogen (n‐Bu 3 SnH) was stirred for 4 hours, purified, and dried to obtain dehydroxylated cephalotaxin norditerpene. The chemical conversion process of the tricuspid norditerpenoids is shown below.
[0065]
[0066] The chemical structure of Gongshan harringtonide I (1) is:
[0067]
[0068] The physical and chemical properties of Gongshan harringtonide I are as follows: light yellow powder; molecular formula C 19 h 20 o 3 ; UV (MeOH) λ max 243, 325nm; hydrog...
preparation Embodiment 1
[0172] Prepare the above-mentioned compound of the present invention according to the method of Preparation Example 1, and use ethanol, glycerin, polyethylene glycol and other cosolvents to dissolve, and make oral preparations; or add water for injection according to routine, fine filter, potting and sterilization preparation into injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com