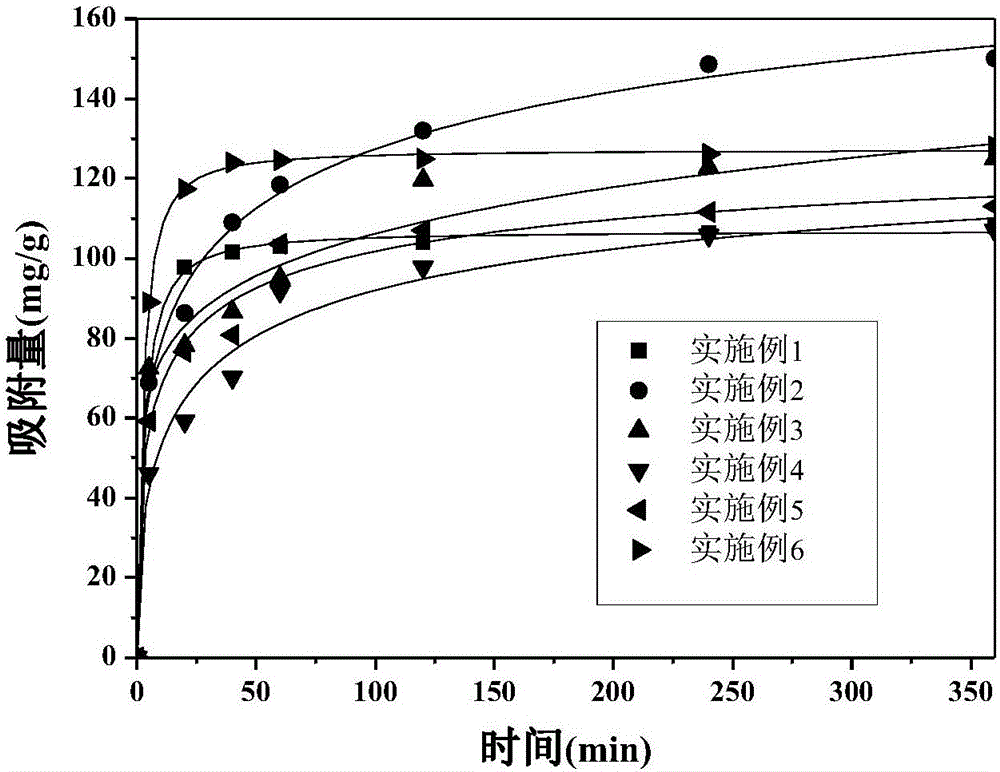
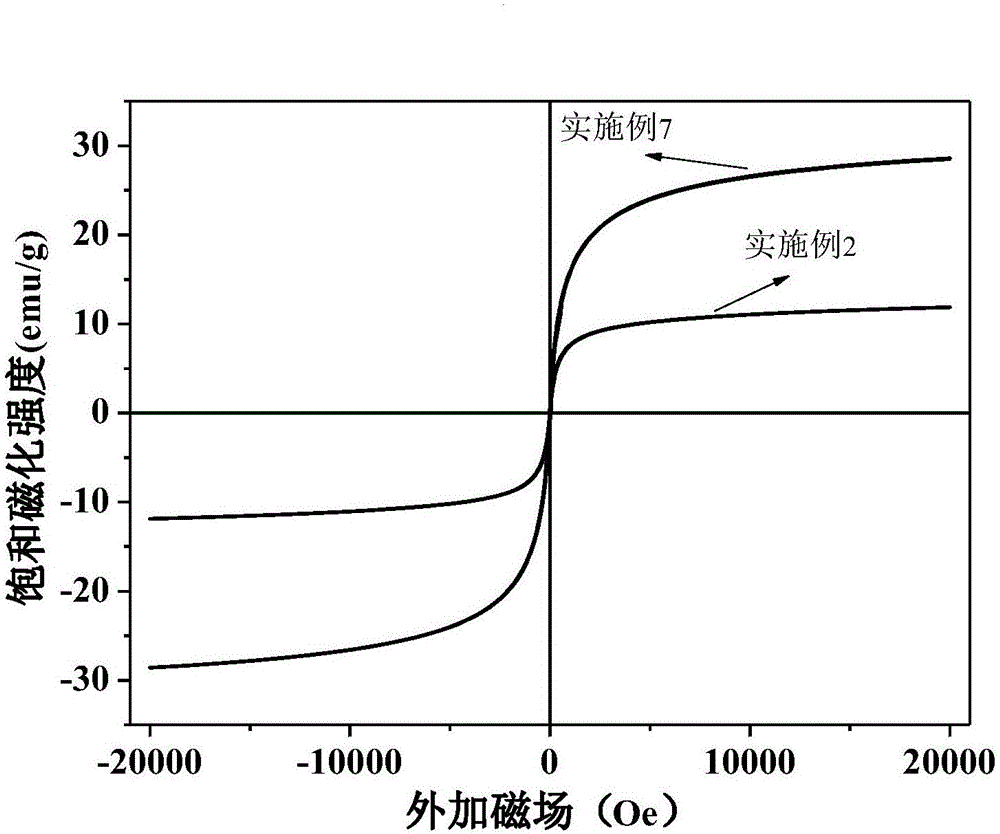
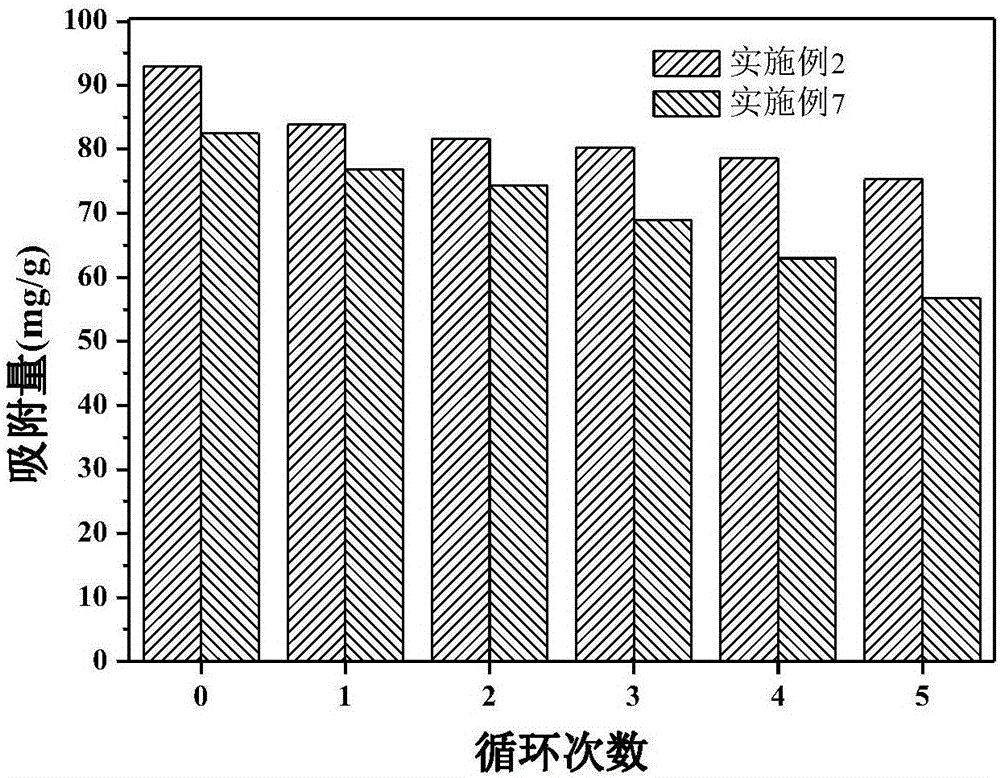
Method for preparing magnetic chitosan adsorbent through Fe3O4/chitosan coprecipitation
A chitosan and adsorbent technology, applied in the field of preparation of magnetic chitosan, can solve the problems of difficult separation, reduce the adsorption capacity of chitosan, etc., and achieve stable chemical properties, good recycling regeneration adsorption performance, and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1mmol FeCl 3 ·6H 2 After O was dissolved in 50ml of deionized water, 1g of chitosan was added and stirred at room temperature for 2h; then 1mmol of FeCl was added 2 4H 2 O, continue stirring for 2h; then slowly add 100ml, a mass fraction of 99.5% absolute ethanol and 10ml, a mass fraction of 25% ammonia water for precipitation; After washing with absolute ethanol, after repeating three times, the product was dried under vacuum conditions at 60° C. for 12 h; Carry out cross-linking in a mixed solution of water and ethanol, stir at room temperature for 2 hours; then undergo magnetic separation and wash with absolute ethanol with a mass fraction of 99.5%, repeat three times, and then dry the obtained product under vacuum at 60°C for 12 hours , to obtain magnetic chitosan.
[0035] When adsorbing 100ml, 200mg / L of Cr(VI) solution, adjust its pH to 2 with a hydrochloric acid solution with a concentration of 0.1mol / L, then add 0.1g of magnetic chitosan sample, and set the...
Embodiment 2
[0038] 1mmol FeCl 3 ·6H 2 After O was dissolved in 50ml of deionized water, 1g of chitosan was added and stirred at room temperature for 2h; then 0.5mmol of FeCl was added 2 4H 2 O, continue stirring for 2h; then slowly add 100ml, a mass fraction of 99.5% absolute ethanol and 10ml, a mass fraction of 25% ammonia water for precipitation; Washing with absolute ethanol was repeated three times, and the product was dried under vacuum at 60°C for 12 hours; after grinding the obtained product, it was dispersed into 10ml of glutaraldehyde with a volume fraction of 25% and 40ml of anhydrous Carry out cross-linking in a mixed solution of ethanol, stir at room temperature for 2 h; then undergo magnetic separation and wash with absolute ethanol with a mass fraction of 99.5%, repeat three times, and then dry the obtained product under vacuum at 60°C for 12 h, namely Obtain magnetic chitosan.
[0039] When absorbing 100ml, 200mg / L Cr(VI) solution, adjust the pH to 2 with a hydrochloric...
Embodiment 3
[0042] 2mmol FeCl 3 ·6H 2 After O was dissolved in 50ml of deionized water, 1g of chitosan was added and stirred at room temperature for 2h; then 0.5mmol of FeCl was added 2 4H 2 O, continue to stir for 2h; then slowly add 100ml, a mixed solution of absolute ethanol with a mass fraction of 99.5% and 10ml, a mixed solution of ammonia with a mass fraction of 25% for precipitation; the obtained precipitate is magnetically separated with a concentration and mass fraction of 99.5% Washing with absolute ethanol, repeated three times, then the product obtained was dried under vacuum conditions at 60°C for 12h; after the product obtained was ground, it was dispersed into 10ml of glutaraldehyde with a volume fraction of 25% and 40ml with a mass fraction of Carry out cross-linking in a mixed solution of 99.5% absolute ethanol, stir at room temperature for 2 hours; then undergo magnetic separation and wash with 99.5% absolute ethanol, repeat three times, and then vacuum-dry the obtaine...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Adsorption capacity | aaaaa | aaaaa |
| Adsorption capacity | aaaaa | aaaaa |
| Adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com