Coumarin phenyl isoxazole derivatives and applications thereof
A technology of phenylisoxazole and isoxazole, which can be applied in drug combinations, skin diseases, and vector-borne diseases, etc., can solve the constraints of secondary development, weak material basis research, and inconsistencies in the mechanism of action and metabolic processes in vivo and in vitro. Clarity and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
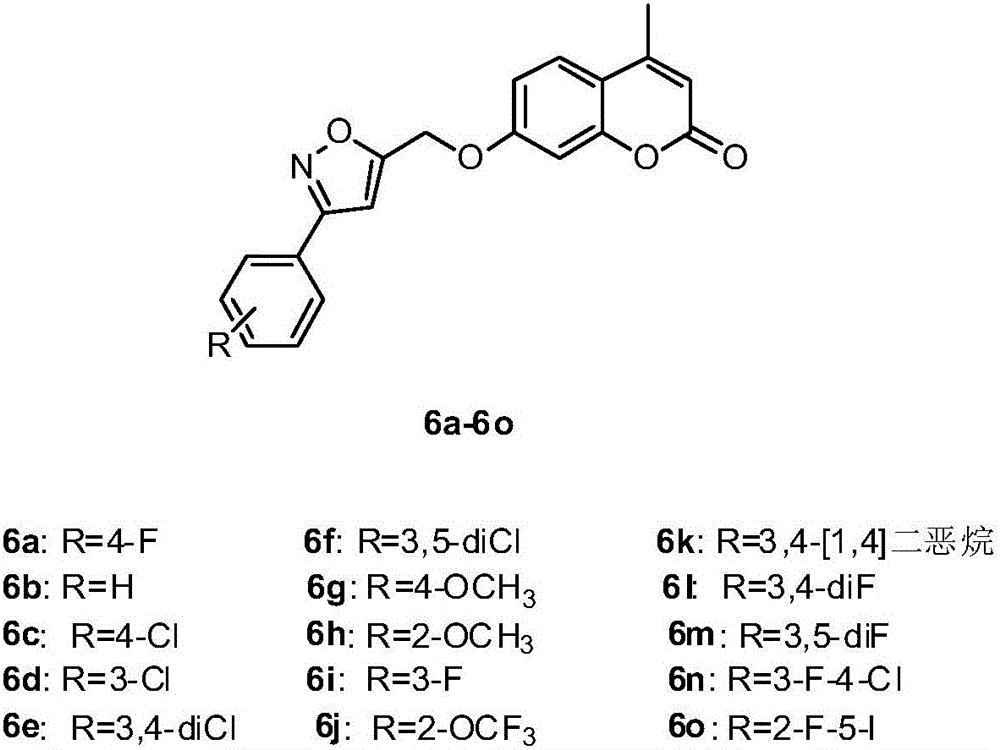
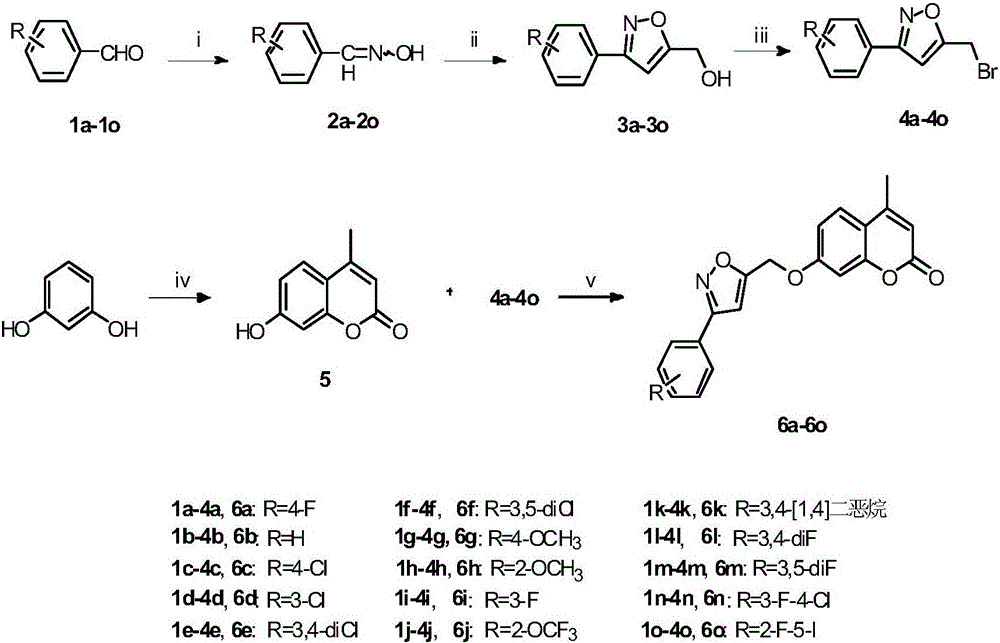
[0063] Preparation of 3-substituted phenyl-5-bromomethylisoxazoles (4a-4o):
[0064] 1.1 Synthesis of substituted benzaldoxime (2a-2o):
[0065] Dissolve 10.0 mmol of benzaldehyde containing different R substitutions in 30 mL of 30% aqueous methanol, and add 695 mg (10.0 mmol) of NH 2 OH·HCl, slowly add 530mg (5.0mmol) Na after dissolving 2 CO3 , react at room temperature for 2h, then add 30mL distilled water and 30mL dichloromethane to extract 3 times, combine the organic phases, and use anhydrous Na 2 SO 4 Dry, filter, and evaporate the solvent under reduced pressure to obtain the corresponding substituted benzaldoxime 2a-2o, which is directly carried out to the next step without separation and purification;
[0066] 1.2 Synthesis of 3-substituted phenyl-5-hydroxymethylisoxazole (3a-3o):
[0067] Dissolve 10.0 mmol of benzaldoxime (2a-2o) containing different R substitutions in 30 mL of dichloromethane, add 1.60 g (12.0 mmol) of N-chlorosuccinimide (NCS) in batches under...
Embodiment 2
[0071] The preparation of 4-methylumbelliferone (compound 5):
[0072] Under ice-bath conditions, 2.0 g (18.2 mmol) of resorcinol was dissolved in dioxane solution, and 0.5 ml of concentrated sulfuric acid was slowly added dropwise, and the temperature of the reaction solution did not exceed 20°C. After the dropwise addition is complete, continue to add 2.8 g (21.8 mmol) of ethyl acetoacetate to the reaction solution, and heat to 60°C. After TLC detects that all the raw materials disappear, the reaction solution is poured into ice water, and a large amount of solid is precipitated, filtered, and dried. The crude product was obtained, and 2.87 g of 4-methylumbelliferone (compound 5) was obtained after methanol recrystallization.
Embodiment 3
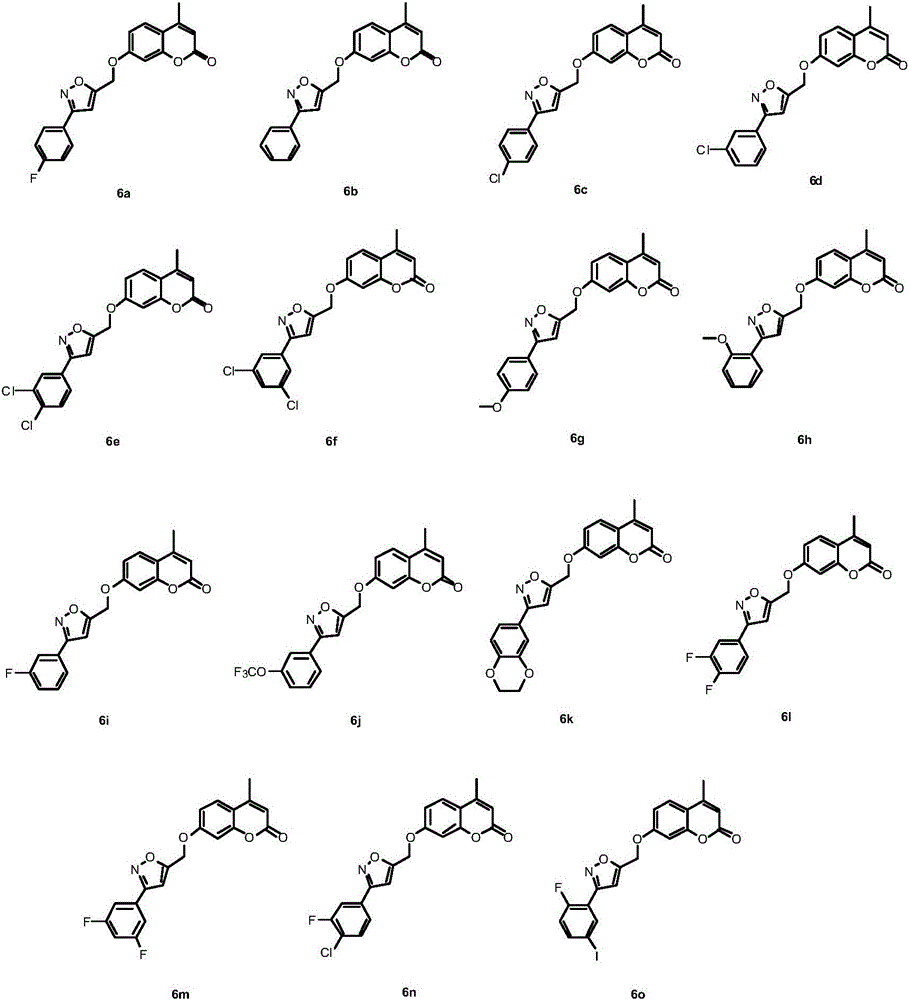
[0074] Preparation of 7-((3-(4-fluorophenyl)isoxazol-5-yl)methoxy)-4-methyl-2H-benzopyran-2-one (compound 6a):
[0075] At room temperature, 0.176g (1mmol) 4-methylumbelliferone, 0.307g (1.2mmol) 3-(4-fluoro)phenyl-5-bromomethylisoxazole and 0.414g (3.0mmol) Dissolve dry potassium carbonate in 50ml of dry acetone, heat it to a temperature of 56°C, and allow it to reflux for 4 hours. After the reaction is complete by TLC, stop the reaction and filter, and the filtrate is vacuum-desolvated. : 1 petroleum ether: ethyl acetate column chromatography with gradient elution to obtain 0.284 g of compound 6a.
[0076] NMR data of 7-((3-(4-fluorophenyl)isoxazol-5-yl)methoxy)-4-methyl-2H-benzopyran-2-one (compound 6a):
[0077] 1 H NMR (400MHz, CDCl 3 )δ7.83-7.76(m,2H),7.55(d,J=8.6Hz,1H),7.15(t,J=8.6Hz,2H),6.98-6.91(m,2H),6.66(s,1H ),6.18(s,1H),5.27(s,2H),2.41(s,3H).
[0078] 13 C NMR (101MHz, CDCl 3 )δ 167.37, 165.19, 162.70, 160.46, 155.12, 152.31, 130.91, 128.82, 125.92, 116.15,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com