Kit for rapidly diagnosing lipoprotein-associated phospholipase A2 and use method of kit
A rapid diagnosis, lipoprotein technology, applied in the field of medical biological immunological detection reagents, can solve the problems of unstable material properties, low sensitivity, unsuitable for large batches, automatic detection, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1 Composition and preparation of detection kit of the present invention
[0048] The test kit of the present invention comprises the following components:
[0049] Reagent a: standard and quality control
[0050] Reagent b: liposome complex
[0051] Reagent c: Magnetic separation reagent
[0052] Reagent d: Assay buffer
[0053] Reagent e: cleaning solution
[0054] The preparation of each reagent in the kit of the present invention comprises the following steps:
[0055] (1) Preparation of lipoprotein-associated phospholipase A2 standard substance and quality control substance
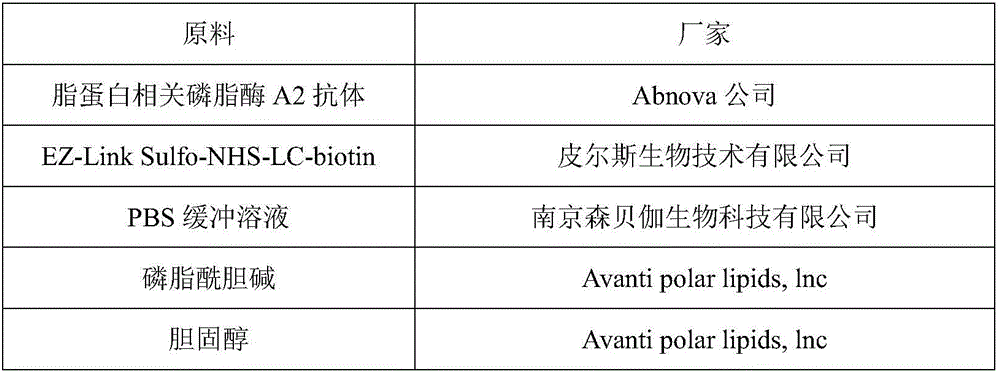
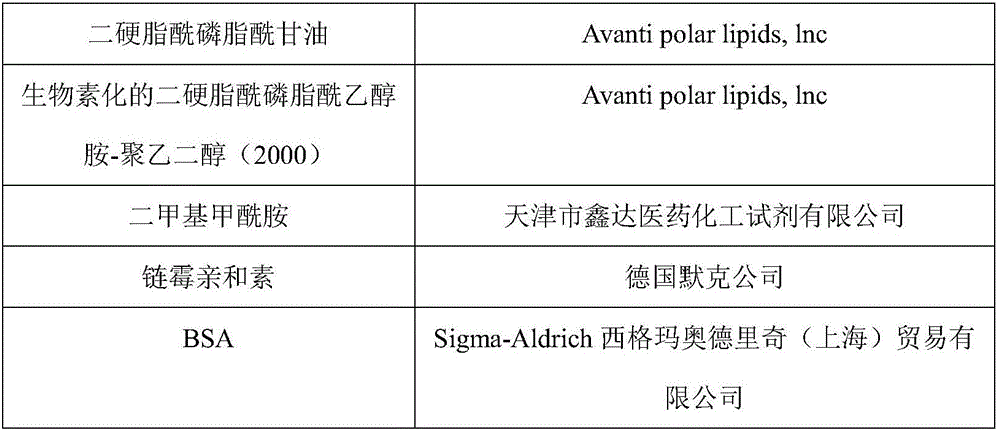
[0056] raw material factory Potassium dihydrogen phosphate Shenzhen Excellence Biotechnology Co., Ltd. Sodium chloride Shenzhen Excellence Biotechnology Co., Ltd. BSA Sigma-Aldrich Sigma-Aldrich (Shanghai) Trading Co., Ltd. Tween-20 Shanghai McLean Biochemical Technology Co., Ltd. lipoprotein-associated phospholipase A2 antigen Abnov...
Embodiment 2
[0086] Example 2 Using the detection kit of the present invention to detect Lp-PLA2
[0087] (1) Preparation of the sample to be tested: the collection of the sample refers to the routine operation, specifically adding the collected venous blood into a test tube or a heparin anticoagulant tube, centrifuging, and taking the supernatant for the experiment.
[0088] (2) Dilute the liposome complex at a ratio of 1:5 with PBS buffer containing 2% (w / v) BSA.
[0089] (3) Take 25 μL diluted liposome complex, 25 μL magnetic separation reagent, 50 μL sample to be tested or lipoprotein-related phospholipase A2 standard, mix, vortex at room temperature for 1 hour, fully react, and form magnetic particles- For the antigen-liposome complex, the reaction solution is sucked into the electrochemical reaction cell, the magnetic particle-antigen-liposome complex is magnetically adsorbed on the electrode surface, and 100 μL of cleaning solution is added to gently rinse the electrode surface for ...
Embodiment 3
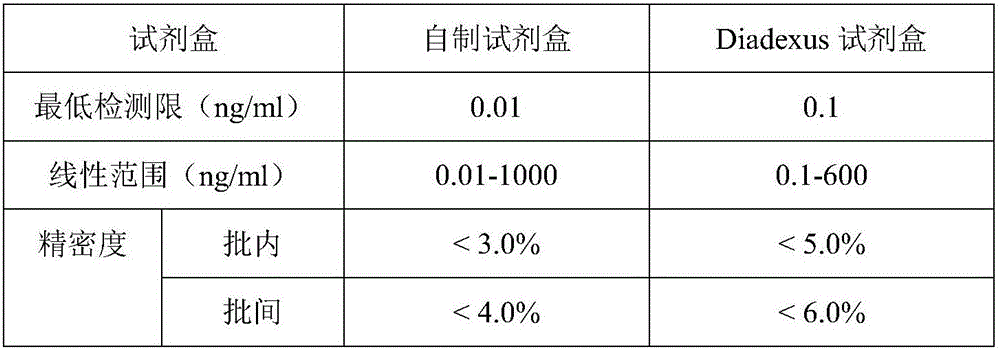
[0093] Embodiment 3 The methodological examination of detection kit of the present invention
[0094] The detection kit prepared in the embodiment is tested according to the routine manufacturing and testing procedures in the art, and the results are as follows:
[0095] ① Analytical sensitivity
[0096] Repeat the determination of the zero calibration product (or sample diluent) for no less than 10 times, calculate the mean (x) and standard deviation (s) of the response, and substitute the response of (x+2s) into the dose-response curve to calculate The corresponding concentration value is the minimum detection limit, and the minimum detection limit of the self-made kit obtained is 0.01ng / ml.
[0097] ②Linear relationship
[0098] Taking the logarithm of the concentration of the reference standard in the self-made kit as the abscissa, and the logarithm of the signal value count as the ordinate, the log-log function of the double-logarithmic mathematical model is used to mea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com