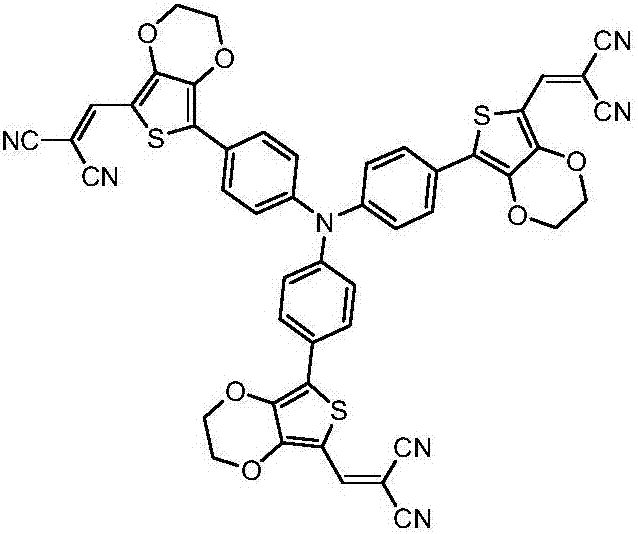
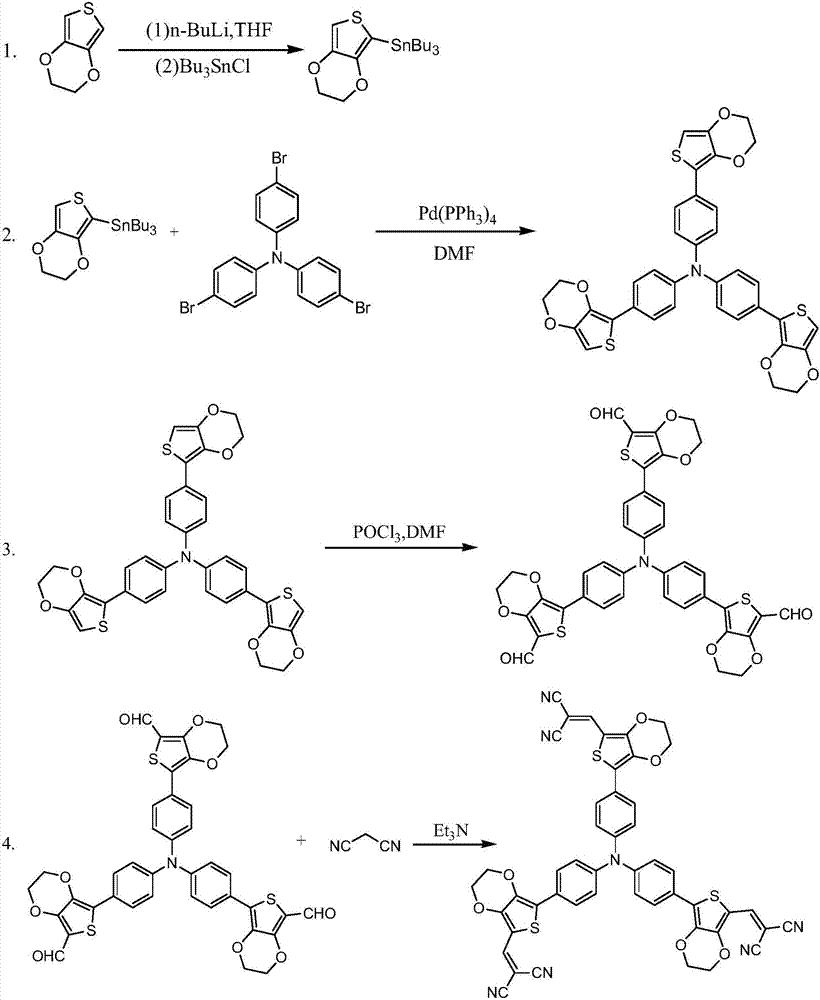
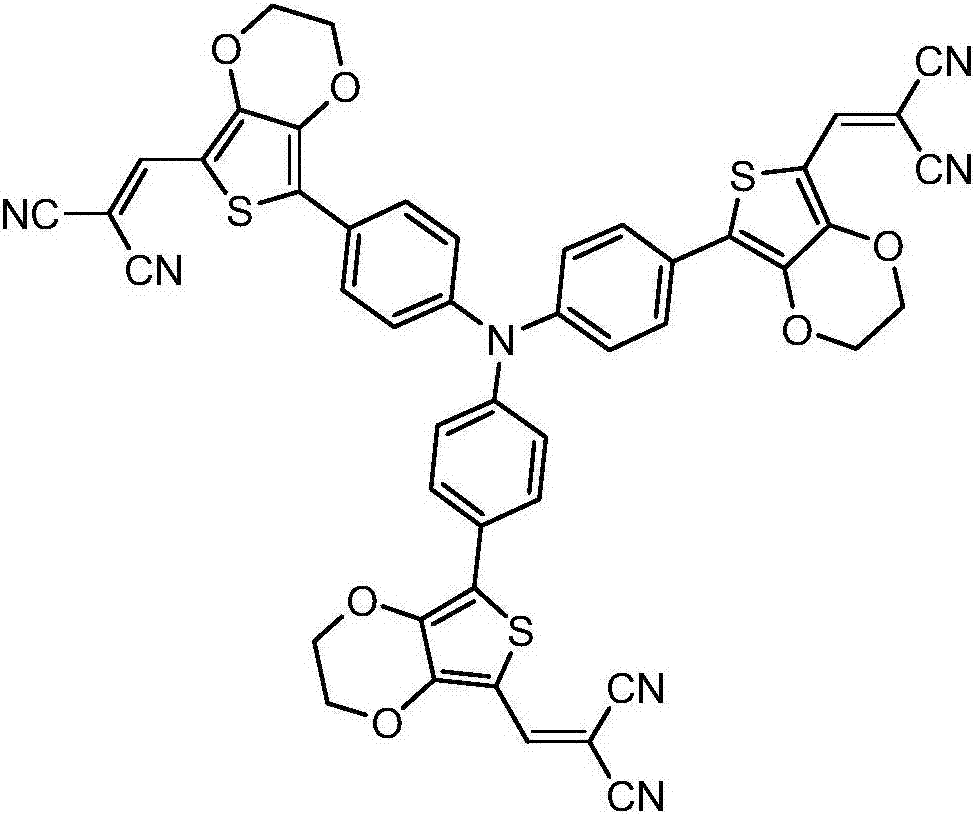
Star-shaped triphenylamine derivative, and preparation method and applications thereof
A technology of triphenylamine and its derivatives, which is applied in the field of organic optoelectronic materials, can solve the problems that molecules are not easy to pile up in an orderly manner, and the photoelectric conversion efficiency is not as good as that of molecules with symmetrical structures, and the difference is far away, so as to facilitate transmission and separation, and improve photoelectric conversion. Efficiency, the effect of increasing absorbance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Step 1: Add 13.93mmol of 3,4-ethylenedioxythiophene and 80ml of tetrahydrofuran into the reaction vessel under a nitrogen atmosphere, cool down to -78°C, then slowly add 15.32mmol of n-butyllithium dropwise, and react for 20 minutes Return to room temperature naturally, continue stirring for 1 h, then lower the temperature to -78°C, then slowly add 15.32 mmol of tributyltin chloride dropwise, return to room temperature naturally, and stir overnight. After the reaction was completed, it was quenched with 30 ml of ice water, extracted with dichloromethane, washed twice with saturated sodium chloride solution and distilled water, dried by adding anhydrous sodium sulfate, and concentrated by suspension evaporation. The obtained crude product was purified by silica gel column chromatography, gradient elution, and the solvent was removed to obtain an intermediate product 1: 2-(tributyltin)-3,4-ethylenedioxythiophene.
[0028] MS: m / z=432.15
[0029] 1 H NMR (300 MHz, DMSO) ...
Embodiment 2
[0044] Step 1: Add 27.86mmol of 3,4-ethylenedioxythiophene and 90ml of tetrahydrofuran into the reaction vessel under a nitrogen atmosphere, cool down to -78°C, then slowly add 30.65mmol of n-butyllithium dropwise, and react for 40 minutes Return to room temperature naturally, continue stirring for 2 h, then lower the temperature to -78°C, then slowly add 30.65 mmol of tributyltin chloride dropwise, return to room temperature naturally, and stir overnight. After the reaction was completed, it was quenched with 35 ml of ice water, extracted with dichloromethane, washed twice with saturated sodium chloride solution and distilled water, dried by adding anhydrous sodium sulfate, and concentrated by suspension evaporation. The obtained crude product was purified by silica gel column chromatography, gradient elution, and the solvent was removed to obtain an intermediate product 1: 2-(tributyltin)-3,4-ethylenedioxythiophene.
[0045] MS: m / z=432.08
[0046] 1 H NMR (300 MHz, DMSO) ...
Embodiment 3
[0061] Step 1: Add 41.79mmol of 3,4-ethylenedioxythiophene and 100ml of tetrahydrofuran into the reaction vessel under a nitrogen atmosphere, cool down to -78°C, and then slowly add 45.969mmol of n-butyllithium dropwise. After 1 hour of reaction, the Return to room temperature, continue stirring for 3 h, then lower the temperature to -78°C, then slowly add 45.97 mmol of tributyltin chloride dropwise, return to room temperature naturally, and stir overnight. After the reaction was completed, it was quenched with 40 ml of ice water, extracted with dichloromethane, washed three times with saturated sodium chloride solution and distilled water, dried with anhydrous sodium sulfate, and concentrated by suspension evaporation. The obtained crude product was purified by silica gel column chromatography, gradient elution, and the solvent was removed to obtain an intermediate product 1: 2-(tributyltin)-3,4-ethylenedioxythiophene.
[0062] MS: m / z=432.11
[0063] 1 H NMR (300 MHz, DMSO...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com