Synthetic method of artificial florfenicol amine antigen
The technology of florfenicol and artificial antigen is applied in the field of synthesis of florfenicol artificial antigen, which can solve the problems of physical health threat, florfenicol residue and the like, and achieve the effect of simple synthesis steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
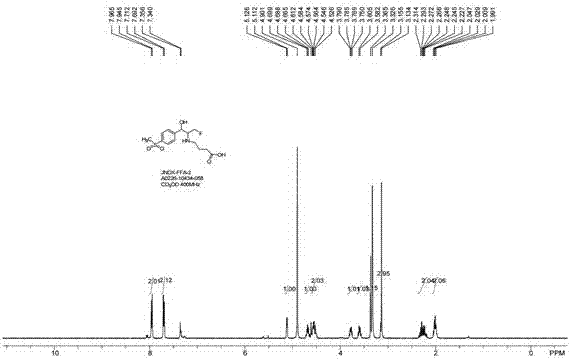
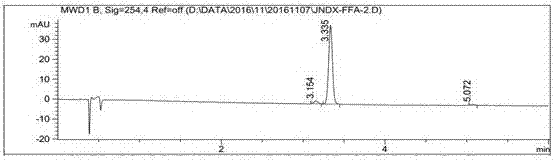
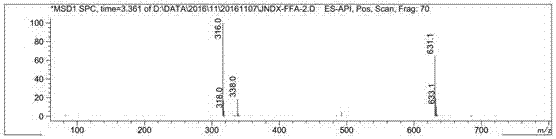
[0032] Example 1 Synthesis of Hapten FFA-2
[0033]
[0034] Weigh 10.0g (28.0mmol) FF and dissolve it in 150mL acetic acid, add 120 mL, 20% H 2 SO 4 , then stirred at 110°C for 3h, then cooled to room temperature, and adjusted the pH to 14, the mixture was extracted by extraction absorption (EA) technology, and then Na 2 SO 4 After concentration and drying, 5.0 g (20.2 mmol) of FFA was obtained as a white solid, with a molar yield of 72.4%.
[0035] Weigh 500.0 mg (2.02 mmol) FFA and 335 mg (2.42 mmol) K 2 CO 3 Dissolve in 5 mL of acetonitrile, add 624 mg (2.42 mmol) benzyl 4-bromobutyrate, stir overnight at 50 °C, then pour the mixture into water, extract by extractive absorption (EA) technique, and then use Na 2 SO 4 Concentrate and dry to obtain crude product. Then the crude product was purified by a silica gel column to obtain 805 mg (1.90 mmol) of white solid FFA-1 with a molar yield of 94.0%.
[0036] Weigh 805 mg (1.90 mmol) FFA-1, and add 8.00 mL, 2 M NH 3...
Embodiment 2
[0038] Example 2 The preparation method of the complete antigen FFA-2-KLH is as follows:
[0039] a. Weigh 2.2 mg of FFA-2, 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride 5 mg, N-hydroxysuccinimide prepared in step (1) 3 mg, dissolved in 400 μL of anhydrous N,N-dimethylformamide, stirred at room temperature for 4-5 hours, called A solution. Take 0.735 mL of keyhole limpet hemocyanin KLH (6.8 mg / mL, the molar ratio of FFA-2 to KLH is 6000:1), add an equal volume of boric acid buffer solution, and call it solution B. At room temperature, add solution A to solution B dropwise, and react overnight at room temperature to obtain the conjugate FFA-2-KLH mixture;
[0040] b. Dialysis: take a dialysis bag of 8 cm, boil it in boiling water for 10 minutes, rinse it with deionized water at 50°C for 2 minutes, and store it in deionized water at 4°C for later use; put the conjugate FFA-2-KLH mixture into the dialyzer The bag was dialyzed in 0.01mol / L PBS for 3 days, and the so...
Embodiment 3
[0041] The complete antigen FFA-2-BSA preparation method described in embodiment 3 is as follows:
[0042] a. Weigh 1.5 mg of FFA-2 prepared in step (1), 4 mg of 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride, 2.5 mg of N-hydroxysuccinimide mg, dissolved in 250 μL of anhydrous N,N-dimethylformamide, stirred at room temperature for 4-5 hours, called A solution. Weigh 5 mg of bovine serum albumin BSA (the molar ratio of FFA-2 to BSA is 60:1), dissolve it in 1 mL of sodium carbonate / sodium bicarbonate buffer solution, add an equal volume of boric acid buffer solution, and call it solution B. At room temperature, add solution A to solution B dropwise, and react overnight at room temperature to obtain the conjugate FFA-2-BSA mixture;
[0043] b. Dialysis: Take a dialysis bag of 8 cm, boil it in boiling water for 10 minutes, rinse it with 50°C deionized water for 2 minutes, store it in 4°C deionized water for later use; put the conjugate FFA-2-BSA mixture into the dialy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com