Metal ion liquid absorber having effect of efficiently and reversibly absorbing ammonia gas
A technology of liquid absorbent and metal ions, which is applied in gas treatment, separation methods, and separation of dispersed particles, etc., can solve the problems of poor heat transfer performance and circulation, and achieve improved absorption performance, high absorption capacity, and good stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
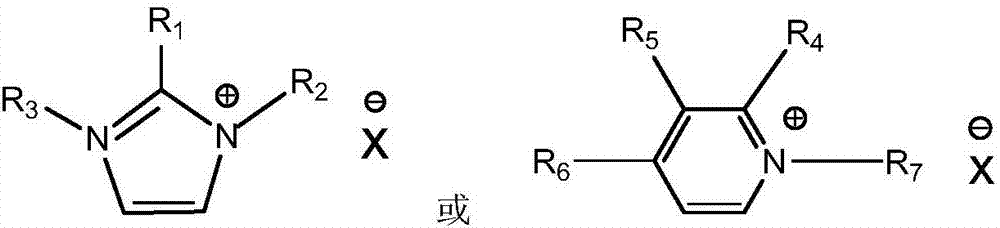
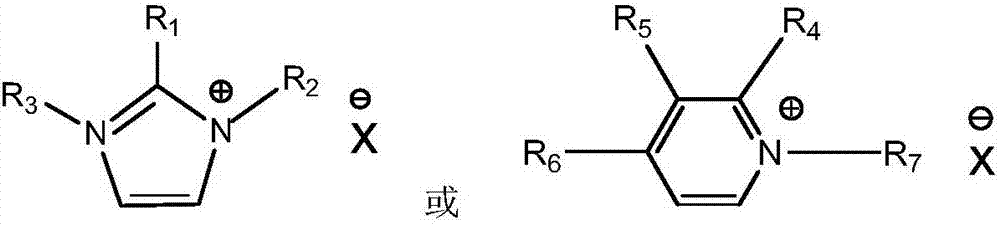
[0014] 1) Add 1mol of N-methylimidazole into a 500mL three-necked round-bottomed flask with a condenser, stir it magnetically to raise the reaction temperature to 65°C, then slowly add 1.05mol of n-chlorobutane dropwise, dropwise After the addition, the temperature was raised to 70°C, and the reaction was heated to reflux for 48h. Take out and extract three times with ethyl acetate, and the ionic liquid phase is removed by a rotary evaporator to remove residual organic solvents to obtain a white waxy solid [Bmim][Cl] at room temperature. Take [Bmim][Cl] (20.96g, 0.12mol) and anhydrous copper chloride (8.34g, 0.062mol) and stir at 70°C for 24h. After the reaction, wash with acetone, filter and rotary evaporate, and vacuum dry at 70°C for 48 hours to obtain viscous liquid [Bmim] 2 [CuCl 4 ].
[0015] 2) Add 1mol of pyridine into a 500mL three-neck round bottom flask with a condenser, stir it magnetically to raise the reaction temperature to 65°C, then slowly add 1.05mol of n-...
Embodiment 2
[0018] Determination by thermogravimetric analyzer [Bmim] 2 [CuCl 4 ],[Bmim] 2 [SnCl 4 ],[Bmim] 2 [NiCl 4 ], [Bmim][ZnCl 3 ] and [Bmim]Cl five kinds of ionic liquid thermal decomposition temperature, test conditions: from room temperature to 600 ℃, N 2 atmosphere, the heating rate is 10°C / min, and the decomposition temperatures are 220°C, 240°C, 286°C, 331°C and 205°C, respectively, and the thermal stability of the metal ionic liquid is significantly improved.
Embodiment 3
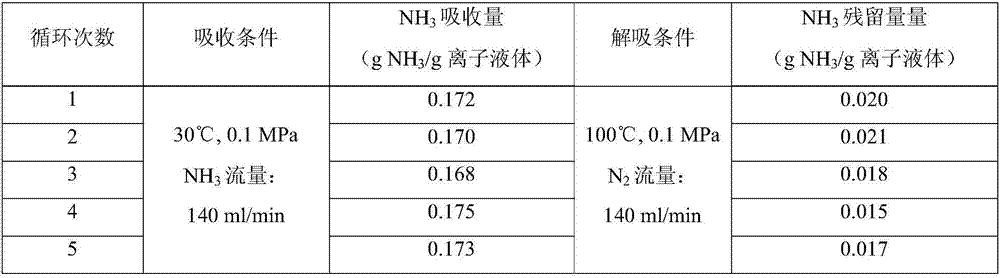
[0020] 1) In a self-made absorption bottle with an internal diameter of 3.00cm, add 5.00g of the ionic liquid [Bmim] synthesized in 1) in Example 1 2 [CuCl 4 ], and then into pure NH 3 , the gas flow rate is 140ml / min, the temperature is 30°C, and the pressure is 0.1MPa. Take the weight of the absorption bottle at regular intervals until the mass does not change, and the absorption reaches equilibrium in about 5 hours. The calculated ammonia absorption capacity in the ionic liquid is 0.172g NH 3 / g ionic liquid (4.892mol NH 3 / mol ionic liquid).
[0021] 2) In the self-made absorption bottle that internal diameter is 3.00cm, add 5.00g embodiment 1 in 2) the ionic liquid [C] synthesized 4 Py] 2 [CuCl 4 ], and then into pure NH 3 , the gas flow rate is 140ml / min, the temperature is 30°C, and the pressure is 0.1MPa. Take the weight of the absorption bottle at regular intervals until the mass does not change, and the absorption reaches equilibrium in about 5 hours. The cal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com