A kind of synthetic method of fluorine-containing acrylate copolymer
A technology of acrylate and synthesis method, applied in the field of polymer material synthesis, can solve the problems of reduced water contact angle of fluorine-containing copolymer, low utilization rate of fluorine-containing monomer, decreased antifouling performance, etc., and achieves improved hydrophobicity and oleophobicity. Antifouling performance, no emulsifier residue, loss reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
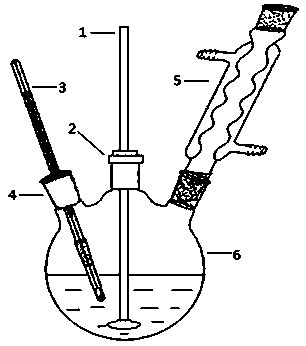
[0038] In the 250ml there-necked flask (its reaction device such as figure 1 As shown), add 0.5g methacryloyloxyethyltrimethylammonium chloride (DMC) as a cationic emulsifier, then add 60ml deionized water, stir evenly at room temperature to fully dissolve DMC.
[0039] Dissolve 0.16 g of azobisisobutyronitrile (AIBN) with a small amount of acetone, then mix to 1 g of hexadecane, 1 g of perfluorooctyl ethyl acrylate (FA), 10 g of butyl methacrylate (BMA), 8 g of formazan In the mixed solution of methyl acrylate (MMA) monomer, the above oil phase mixed solution was slowly added dropwise to the water phase system with a constant pressure dropping funnel, and added dropwise while stirring. Stir mechanically at room temperature for 30 minutes, and then sonicate in an ice-water bath for 5 minutes to obtain a monomer miniemulsion.
[0040] After the system returned to room temperature, the three-neck flask was placed in an electric constant temperature water bath, stirred rapidly (...
Embodiment 2
[0043] In the 250ml there-necked flask (its reaction device such as figure 1 As shown), add 0.5g methacryloyloxyethyltrimethylammonium chloride (DMC) as a cationic emulsifier, then add 60ml deionized water, stir evenly at room temperature to fully dissolve DMC.
[0044] Dissolve 0.16 g of azobisisobutyronitrile (AIBN) with a small amount of acetone, then mix to 1 g of hexadecane, 2 g of perfluorooctyl ethyl acrylate (FA), 10 g of butyl methacrylate (BMA), 8 g of formazan In the mixed solution of methyl acrylate (MMA) monomer, the above oil phase mixed solution was slowly added dropwise to the water phase system with a constant pressure dropping funnel, and added dropwise while stirring. Stir mechanically at room temperature for 30 minutes, and then sonicate in an ice-water bath for 5 minutes to obtain a monomer miniemulsion.
[0045] After the system returned to room temperature, the three-neck flask was placed in an electric constant temperature water bath, stirred rapidly (...
Embodiment 3
[0048] In the 250ml there-necked flask (its reaction device such as figure 1 As shown), add 3g of methacryloxyethyltrimethylammonium chloride (DMC) as a cationic emulsifier, then add 60ml of deionized water, and stir evenly at room temperature to fully dissolve DMC.
[0049] Dissolve 0.32 g of azobisisobutyronitrile (AIBN) with a small amount of acetone, then mix to 1 g of hexadecane, 3 g of perfluorooctyl ethyl acrylate (FA), 10 g of butyl methacrylate (BMA), 8 g of formazan In the mixed solution of methyl acrylate (MMA) monomer, the above oil phase mixed solution was slowly added dropwise to the water phase system with a constant pressure dropping funnel, and added dropwise while stirring. Stir mechanically at room temperature for 30 minutes, and then sonicate in an ice-water bath for 5 minutes to obtain a monomer miniemulsion.
[0050] After the system returned to room temperature, the three-neck flask was placed in an electric constant temperature water bath, stirred rapi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com