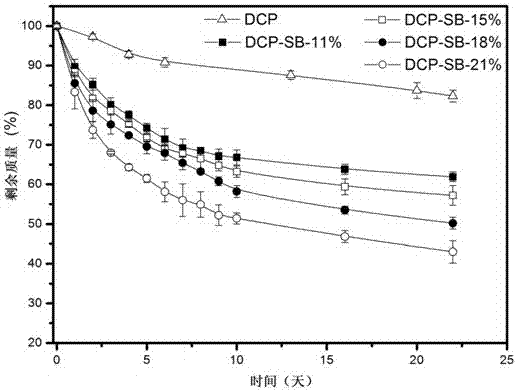
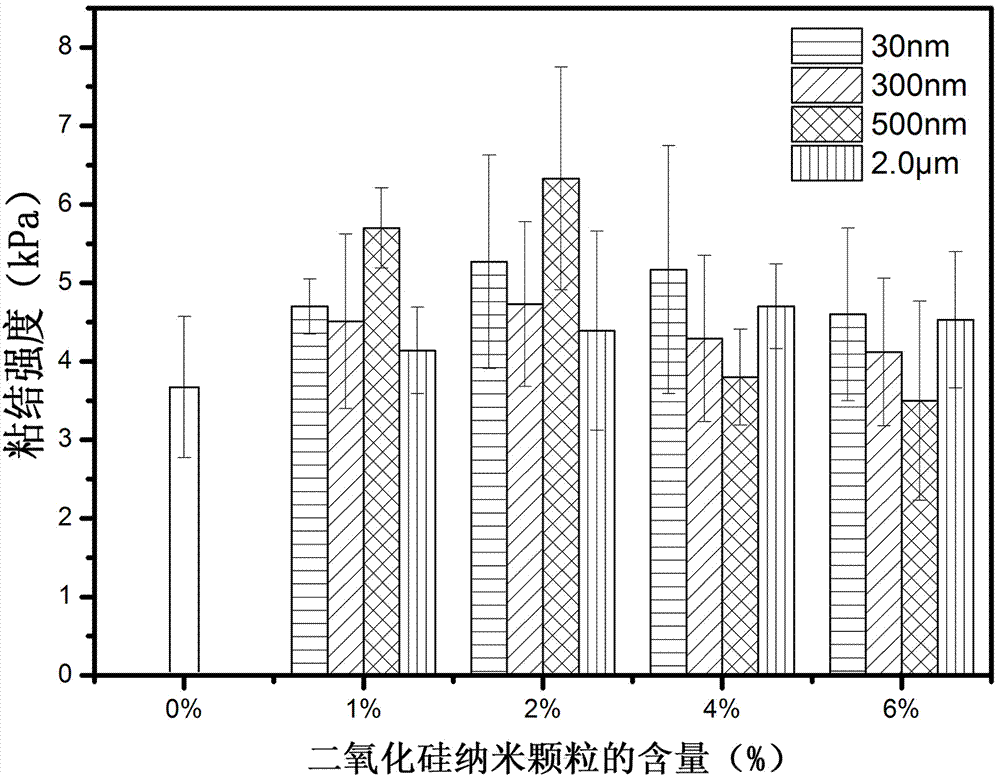
Medical adhesive containing catechol polymer and preparation method thereof
A technology of catechol and polymers, which is applied in the field of medical adhesives containing catechol polymers and its preparation, can solve the problems of low biotoxicity and low degradation rate, and achieve good adhesion tissue effect and low degradation rate. Moderate, simple synthesis and preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] 1. Preparation:
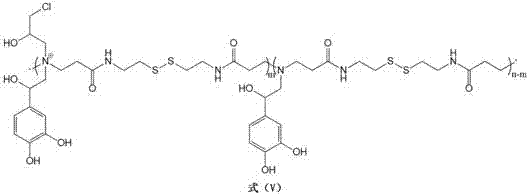
[0051](1) Weigh 1.0 g of dopamine-N,N'-methylenebisacrylamide, 1.2 g of propane sultone, and 0.2 g of sodium bromide in 10 mL of N,N-dimethylformamide (o-phenyl The molar ratio of diphenol repeating units to cyclic monomers was 1:3), sonicated for 20 min to obtain a clear solution, and reacted at 45 °C for 20 h. After the reaction is completed, the resulting mixed system is concentrated by vacuum distillation, and then precipitated twice in tetrahydrofuran to remove unreacted propane sultone, dissolved with N,N-dimethylformamide and transferred to a dialysis bag Dialyzed at 4°C for 2 days, freeze-dried to obtain an improved catechol-containing polymer, whose structural formula is as follows:
[0052]
[0053] (2) Weigh 17 mg of the obtained improved catechol-containing polymer (catechol unit concentration is 2.6 mol / L) and dissolve it in 20 μL dimethyl sulfoxide, sonicate for 20 min, add 1% catechol 500 nm silicon dioxide particles, ultrasonic 10 ...
Embodiment 2
[0058] 1. Preparation
[0059] (1) Weigh 1.0 g of 6-hydroxydopamine-1,6-hexanediol diacrylate copolymer, 0.6 g of styrene oxide, and 0.1 g of lithium bromide in 10 mL of N,N-dimethylacetamide (o-phenyl The molar ratio of diphenol repeating units to cyclic monomers was 1:2), sonicated for 20 min to obtain a clear solution, and reacted at 45 °C for 35 h. After the reaction was completed, the resulting mixed system was concentrated by distillation under reduced pressure, and then precipitated in ethanol twice to remove unreacted lithium bromide. The precipitate was dissolved with N,N-dimethylacetamide and transferred to a dialysis bag for dialysis at 4 °C for 2 days, freeze-dried to obtain an improved catechol-containing polymer, and its structural formula is as follows:
[0060]
[0061] (2) Weigh 21 mg of the obtained improved catechol-containing polymer (catechol unit concentration is 2.6 mol / L) and dissolve it in 20 μL N,N-dimethylacetamide, sonicate for 20 min, add mass ...
Embodiment 3
[0066] 1. Preparation
[0067] (1) Weigh 1.0 g 1-(3,4-dihydroxyphenyl)-2-aminoethanol-N,N'-bis(acryloyl)cystamine copolymer, 0.2 g epichlorohydrin, 0.5 mL Hydrochloric acid was dissolved in 10 mL of N,N-dimethylformamide / water 1:1 mixed solvent (the molar ratio of catechol repeating unit to cyclic monomer was 1:1), and a clear solution was obtained by ultrasonication for 20 min. React at 45 °C for 15 h. After the reaction was completed, the resulting mixed system was concentrated by vacuum distillation, and the precipitate was dissolved with N,N-dimethylformamide, then transferred to a dialysis bag for dialysis at 4 °C for 2 days, and then freeze-dried to obtain an improved catechol-containing A polymer whose structural formula is as follows:
[0068]
[0069] (2) Weigh 23 mg of the obtained improved catechol-containing polymer (catechol unit concentration is 2.6 mol / L) and dissolve it in 20 μL dimethyl sulfoxide, sonicate for 20 min, add 300 nm silica particles, sonicat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com