Bacterium beta-galactosidase as well as encoding gene and application thereof
A technology of galactosidase and coding, which is applied in the field of microorganisms to achieve efficient hydrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
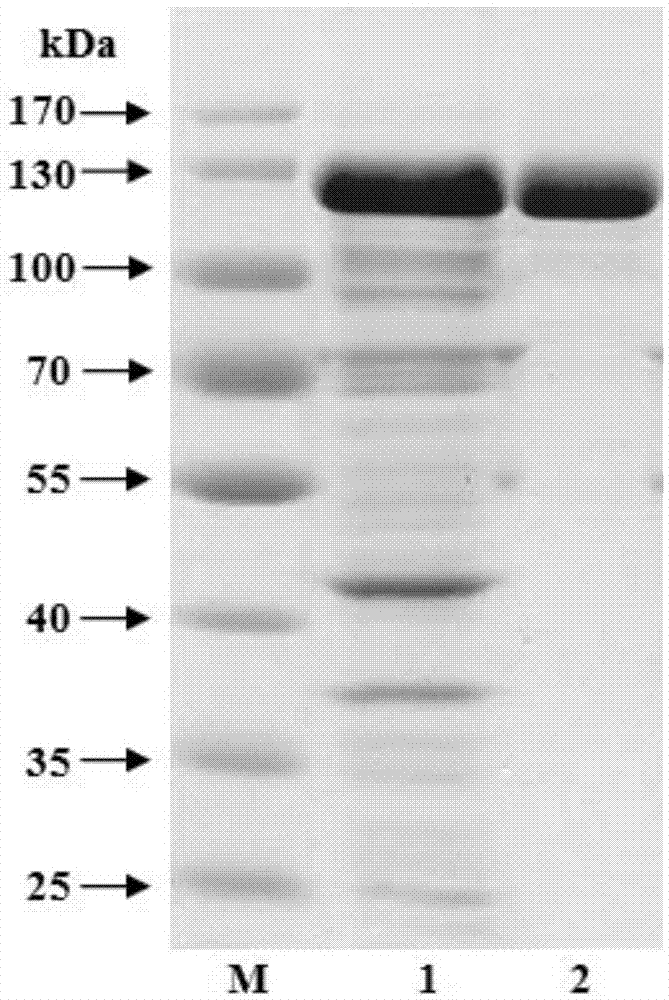
[0066] Embodiment 1, the preparation of recombinant β-galactosidase
[0067] 1. Construction of recombinant β-galactosidase-encoding gene expression plasmid
[0068] 1. Extract the genomic DNA of Paenibacillus barengerzia CAU904 and use it as a template to artificially synthesize PbBgal2ANheF: 5'-ctcag GCTAGC GTGGAACTGAACCGTGAATGG-3' (the underline is the restriction endonuclease NheI site) and PbBgal2AXhoR: 5'-gtcat CTCGAG CTATTCTTCTTCCTTAAATATCGGCTG-3' (the underline is the restriction endonuclease XhoI restriction endonuclease cutting site) is used as primer, carries out PCR amplification, obtains DNA fragment.
[0069] The reaction program was: pre-denaturation at 94°C for 5 min; denaturation at 94°C for 30 s, annealing at 54°C for 30 s, extension at 72°C for 3 min, 35 cycles; extension at 72°C for 5 min.
[0070] 2. Digest the DNA fragment obtained in step 1 with restriction endonucleases NheI and XhoI, and recover the digested product.
[0071] 3. Digest the vector ...
Embodiment 2
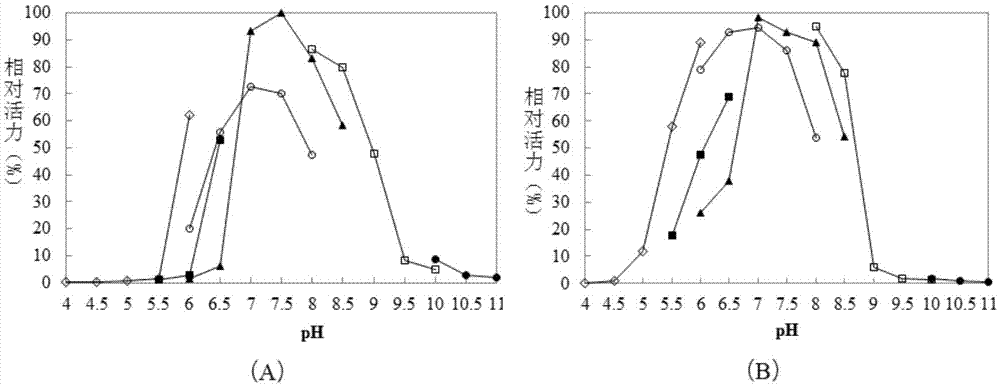
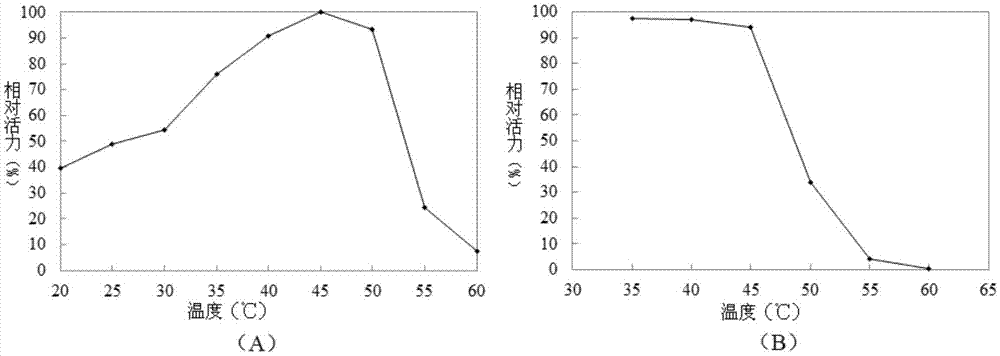
[0084] Embodiment 2, the property of recombinant β-galactosidase
[0085] 1. Optimum pH value and pH stability of recombinant β-galactosidase
[0086] Buffer to be tested (both concentrations are 50mmol / L): acetic acid-sodium acetate buffer solution at pH 4.0, acetic acid-sodium acetate buffer solution at pH 4.5, acetic acid-sodium acetate buffer solution at pH 5.0, and acetic acid-sodium acetate buffer solution at pH 5.5 Acetic acid-sodium acetate buffer, acetic acid-sodium acetate buffer at pH6.0, MES buffer at pH5.5, MES buffer at pH6.0, MES buffer at pH6.5, MOPS buffer at pH6.0, MOPS buffer at pH6.5, MOPS buffer at pH7.0, MOPS buffer at pH7.5, MOPS buffer at pH8.0, sodium dihydrogen phosphate-disodium hydrogen phosphate buffer at pH6.0, pH6. 5 sodium dihydrogen phosphate-disodium hydrogen phosphate buffer, pH7.0 sodium dihydrogen phosphate-disodium hydrogen phosphate buffer, pH7.5 sodium dihydrogen phosphate-disodium hydrogen phosphate buffer, pH8.0 Sodium dihydrogen pho...
Embodiment 3
[0092] Embodiment 3, the application of recombinant β-galactosidase
[0093] TLC detection method: Spot the sample on the TLC analysis plate to develop the layer twice, completely soak it with the color developing solution and dry it, and develop the color at 100°C. The developing agent is n-butanol / ethanol / water=5:3:2 (v / v / v), the chromogenic agent is 5% sulfuric acid methanol solution, and the mixture of standard products of glucose, galactose and lactose is used as standard control.
[0094] HPLC detection method 1: used to determine glucose content, galactose content and lactose content in lactose hydrolysis experiment, the chromatographic column used is cation exchange chromatographic column BP-800Pb 2+ (Benson Polymeric, Reno, NE, USA), the column temperature was 80 °C, the flow rate was 0.6 mL / min, the mobile phase was ultrapure water, and the injection volume was 20 μL. Calculated as follows:
[0095] Glucose content (g / L)=[glucose peak area (mAu*s)-21376] / 458.8 / 100...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com