Patents
Literature
49 results about "Lactose intolerant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
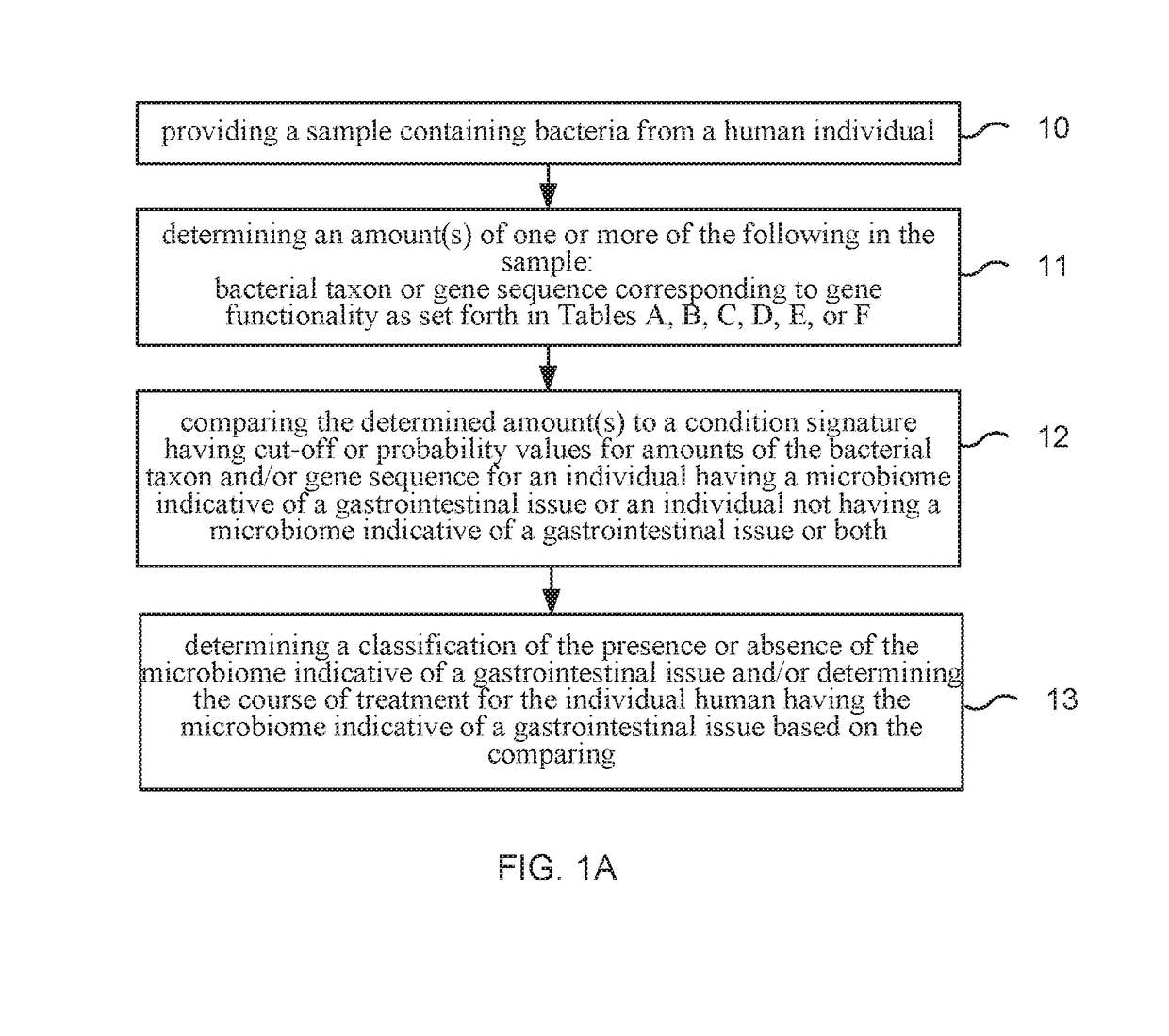
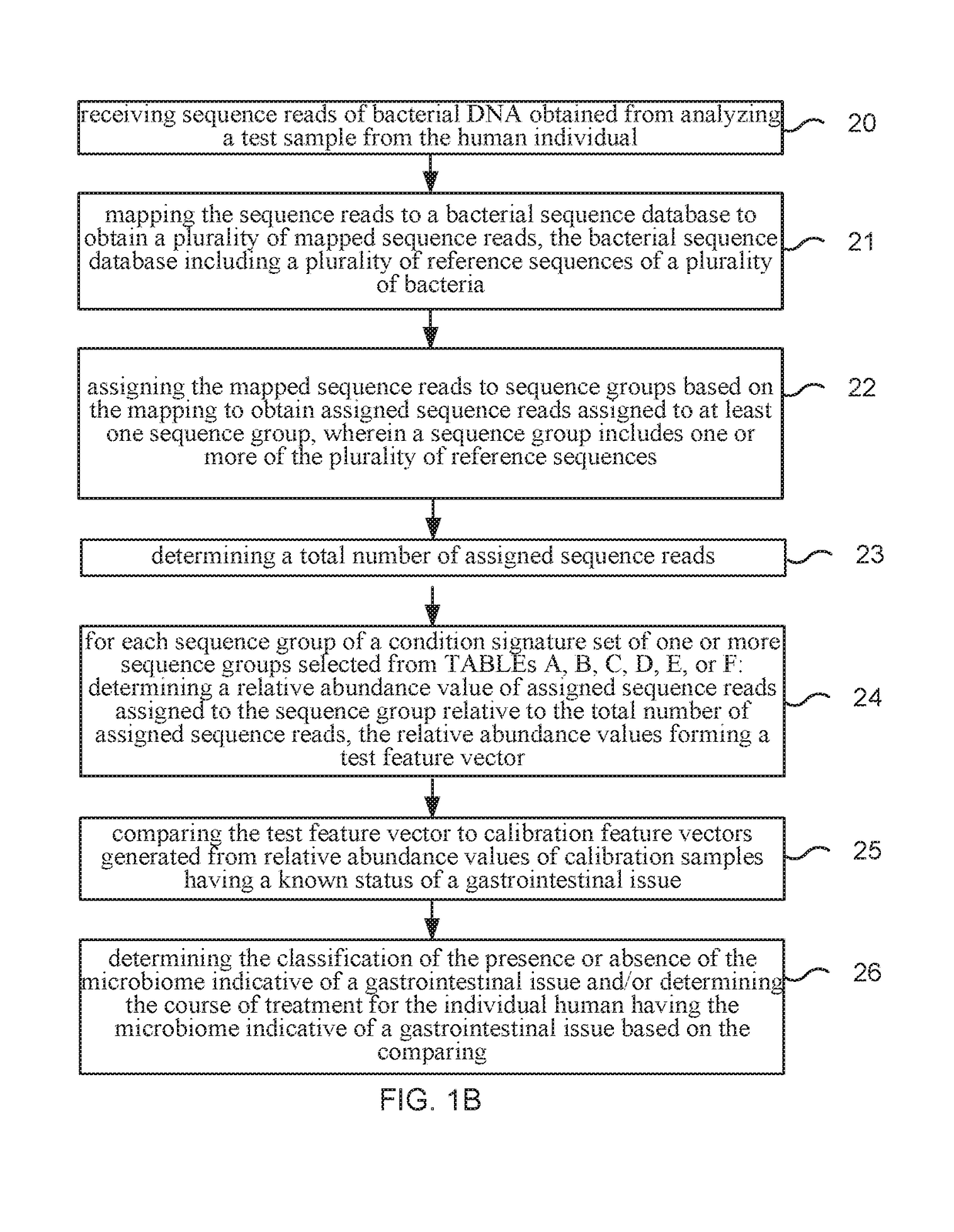
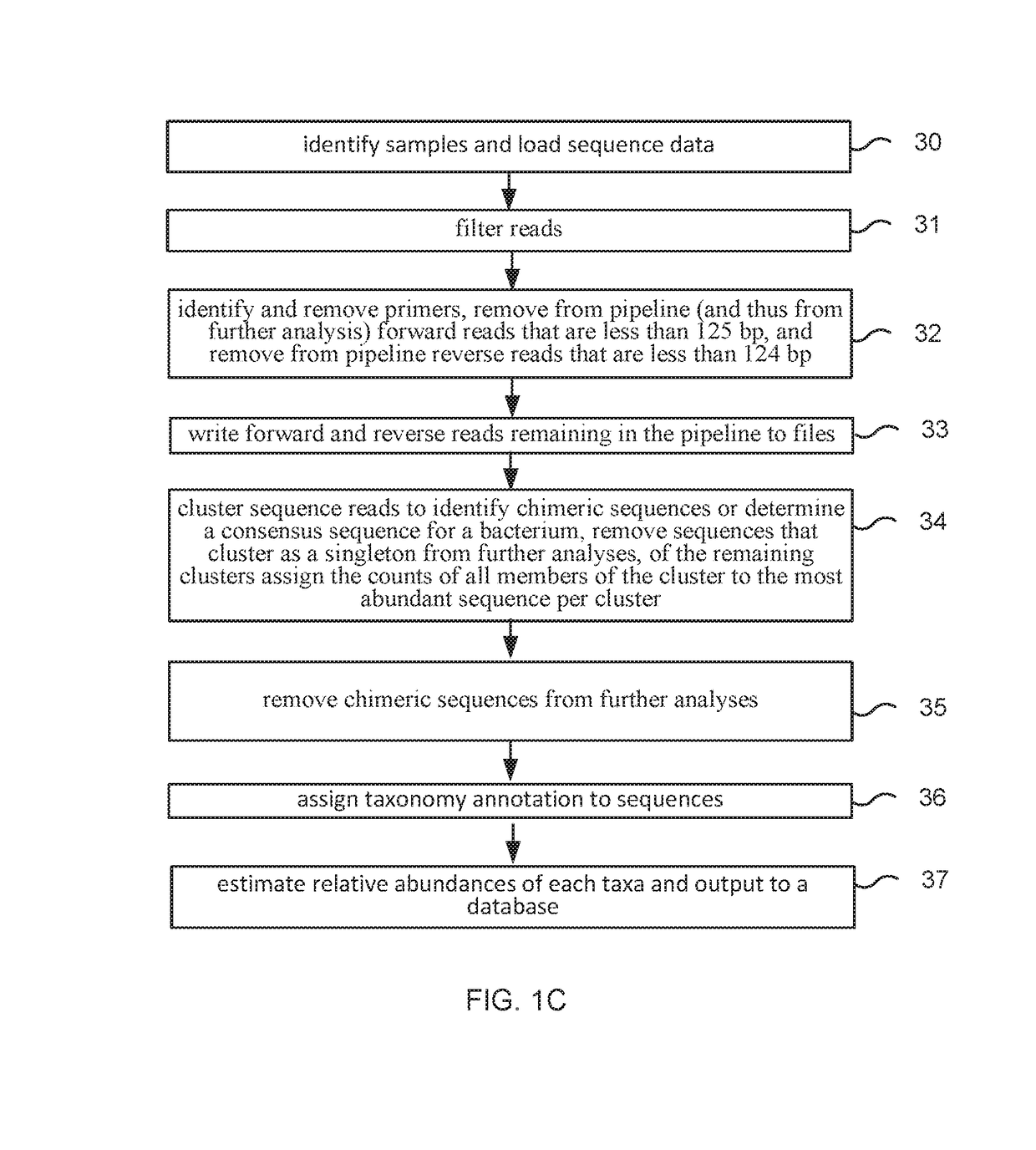
Method and system for microbiome-derived diagnostics and therapeutics for conditions associated with gastrointestinal health
PendingUS20190085396A1Medical simulationMicrobiological testing/measurementLactose intoleranceMedicine
Methods, compositions, and systems are provided for detecting one or more a gastrointestinal issues by characterizing the microbiome of an individual, monitoring such effects, and / or determining, displaying, or promoting a therapy for the gastrointestinal issue. Methods, compositions, and systems are also provided for generating and comparing microbiome composition and / or functional diversity datasets. Methods, compositions, and systems are also provided for generating a characterization model and / or therapy model for constipation issues, diarrhea issues, hemorrhoids issues, bloating issues, and lactose intolerance issues.
Owner:PSOMAGEN INC
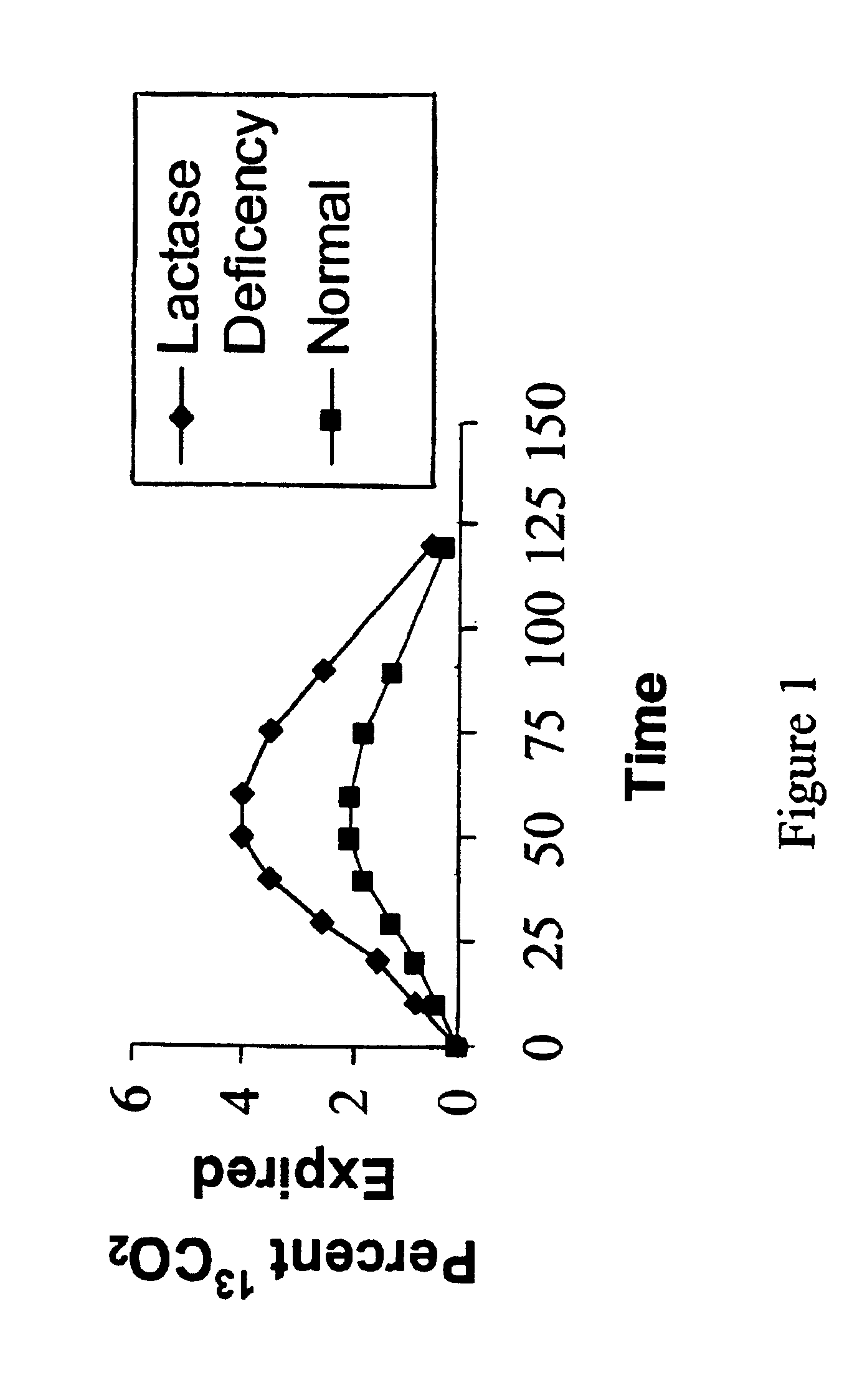
Reverse isotope dilution assay and lactose intolerance assay
InactiveUS6902719B2Cost reductionLow costMicrobiological testing/measurementBiological testingGlucose productionChemistry
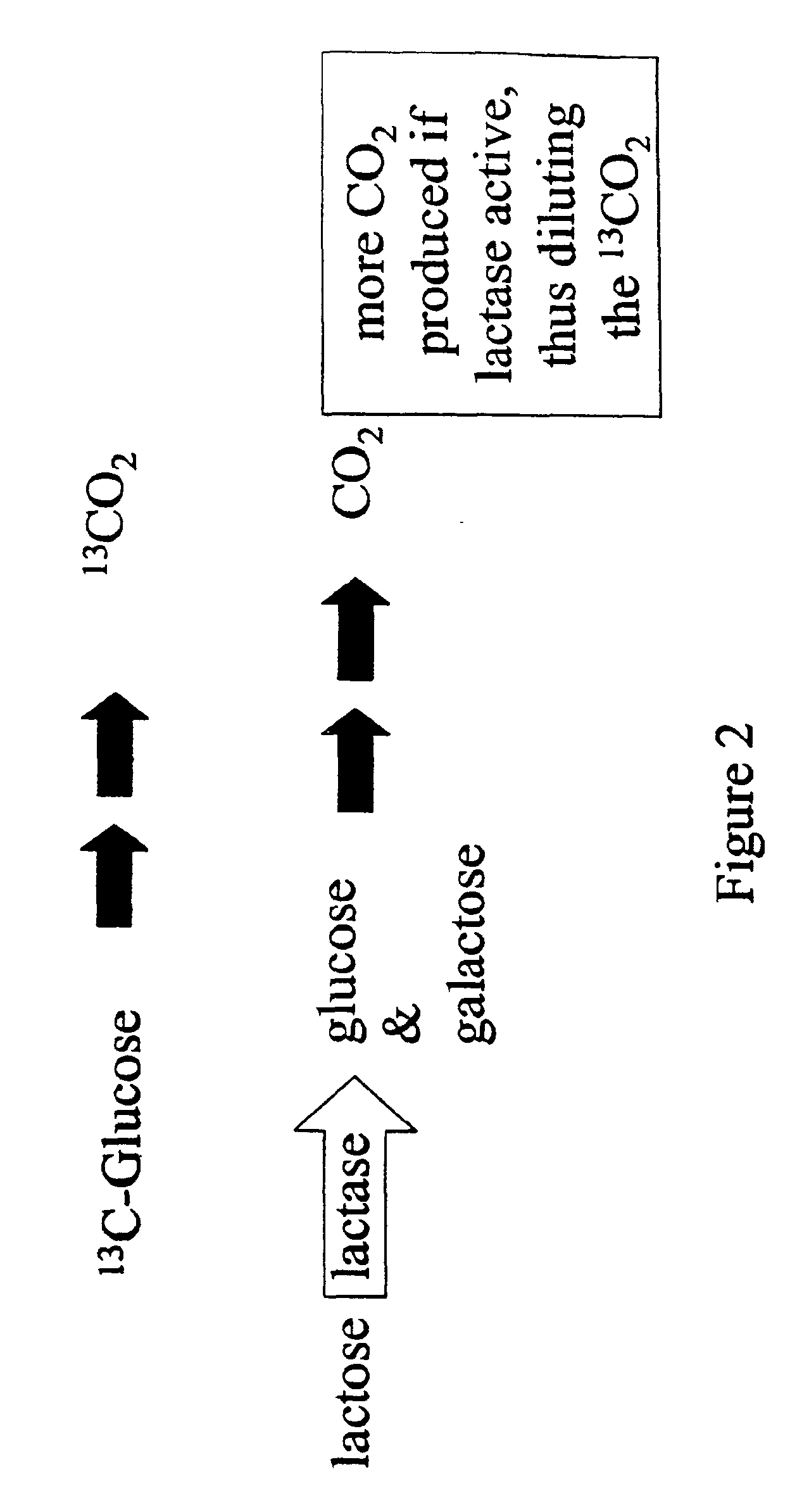
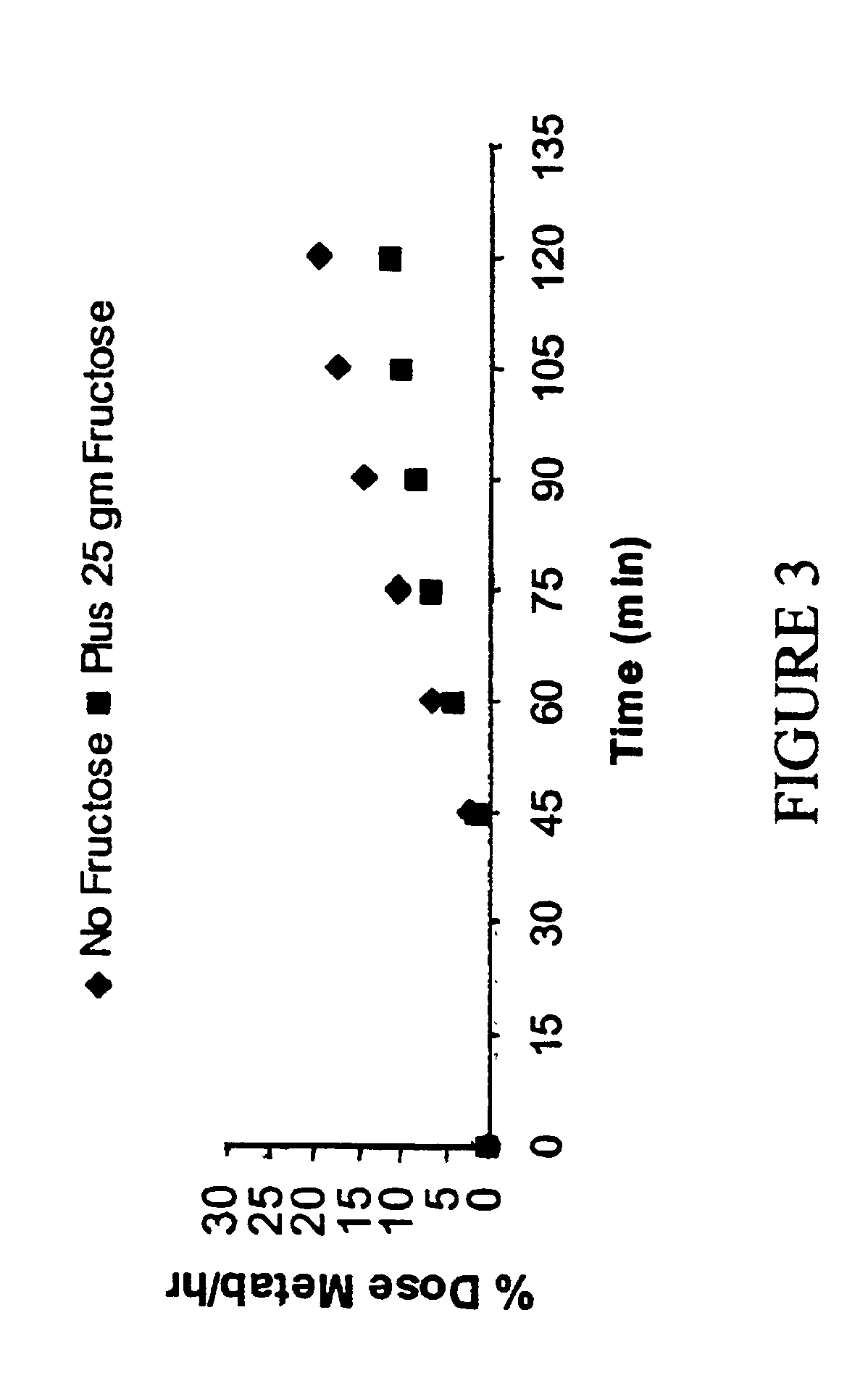
A “reverse isotope dilution assay” herein, wherein a pathway that produces a given metabolite is assayed by diluting a labelled metabolite produced by a second constitutive pathway. In one aspect, the invention relates to a method for monitoring lactose maldigestion or lactose intolerance in humans. Specifically, the method requires administering a reverse tracer of labeled glucose and unlabeled lactose to an individual and assessing labeled carbon dioxide in breath or blood. If the lactose is digested, the labeled CO2 produced by the labeled glucose is diluted by the metabolism of the lactose.
Owner:METABOLIC SOLUTIONS
Lactose-reduced dairy compositions and related methods
Compositions for ameliorating the symptoms associated with lactase deficiency, the composition including a lactose reduced dairy product, and an effective amount of a probiotic, a prebiotic, or a mixture thereof. The lactose reduced dairy product is selected from a fluid milk, a smoothie, a liquado, ice cream, yogurt, and a yogurt drink. Methods for treating lactose intolerance in a patient in need thereof, the method includes providing a composition having a lactose reduced dairy product, and an effective amount of a probiotic, a prebiotic, or a mixture thereof. The lactose reduced dairy product is selected from a fluid milk, a smoothie, a liquado, ice cream, yogurt, and a yogurt drink.
Owner:MCNEIL NUTRITIONALS
Low-lactose milk powder and preparation method thereof
ActiveCN101779703AReduce and improve intolerance symptomsReduce contentMilk preparationFiltrationEvaporation
The invention relates to a low-lactose milk powder and a preparation method thereof. The lactose content of the low-lactose milk powder of the invention is 4.5-10%; the preparation method of the low-lactose milk powder includes that lactose enzyme is added into crude milk for hydrolysis after pasteurization on crude milk and before production burdening on the basis of the conventional production process. The invention adopts advanced biological enzymolysis technology, through researches on technological conditions of the steps of burdening, filtration, homogenizing, preheating and sterilization, evaporation concentration, spray drying, cooling, sieving, packaging and the like, a low lactose milk power is developed, the milk powder is full of nutriments, lactose hydrolysis rate can be above 70%, not only intolerance symptom of lactose intolerant people to lactose can be relived, but also the requirement of the lactose intolerant people on flavour and taste can be met.
Owner:WANDASHAN MILK IND HEILONGJIANG
Method for increasing lactose tolerance in mammals exhibiting lactose intolerance
InactiveUS7029702B2Increasing lactose toleranceImprove toleranceOrganic active ingredientsBiocideTolerabilityMammal
The method for increasing lactose tolerance in subjects exhibiting lactose intolerance symptoms implements a protocol where the subjects ingest a gradually increasing amount of lactose containing product over a six week period. At various points during the six week period the subject ingests the lactose containing product once a day and then twice a day. The lactose containing product can be in liquid form, such as for example, milk, and is preferably in a powder form which is taken either by ingesting capsules having the lactose powder or in a granular form mixed with water or other non-lactose containing liquid. At the end of the six week period, the subject's tolerance for lactose containing products is substantially increased, with the potential of eliminating the subject's lactose intolerant behavior indefinitely.
Owner:RITTER PHARMA
Probiotics enriched crisp apple products and preparation method thereof
The present invention discloses probiotics enriched crisp apple products and a preparation method thereof, and belongs to the technical field of agricultural product processing. Apples are used as raw materials, the apples are washed, the washed apples are blanched, a vacuum soaking method is used to enrich probiotics into apple tissues, and then a vacuum freeze-drying and vacuum microwave combined drying method is used to prepare the probiotics enriched crisp apple products. The method comprises the following steps: (1) raw material washing and blanching; (2) probiotic soaking liquid preparing; (3) vacuum soaking; and (4) vacuum freeze-drying and vacuum microwave combined drying. The prepared probiotics enriched fruit and vegetable crisp products are mellow in fruit fragrance, and crisp and tasty. The viable probiotic number can be maintained at 10<7> CFU / g at 25 DEG C for 90 days or more. The preparation method avoids the two major defects of high cholesterol risk of the probiotic fermented milk products and the non-consumption of lactose intolerant population, and the products combine health care and leisure, and have broad market prospects.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Method for increasing lactose tolerance in mammals exhibiting lactose intolerance
InactiveUS20060104965A1Increasing lactose toleranceImprove toleranceBiocideOrganic active ingredientsTolerabilityEnvironmental health
The method for increasing lactose tolerance in subjects exhibiting lactose intolerance symptoms implements a protocol where the subjects ingest a gradually increasing amount of lactose containing product over a six week period. At various points during the six week period the subject ingests the lactose containing product once a day and then twice a day. The lactose containing product can be in liquid form, such as for example, milk, and is preferably in a powder form which is taken either by ingesting capsules having the lactose powder or in a granular form mixed with water or other non-lactose containing liquid. At the end of the six week period, the subject's tolerance for lactose containing products is substantially increased, with the potential of eliminating the subject's lactose intolerant behavior indefinitely.
Owner:RITTER PHARMA
Plant nutrient powder suitable for babies 12-24 months old
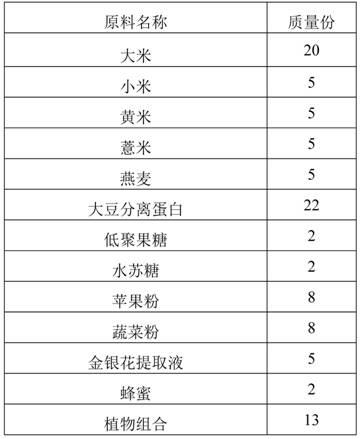
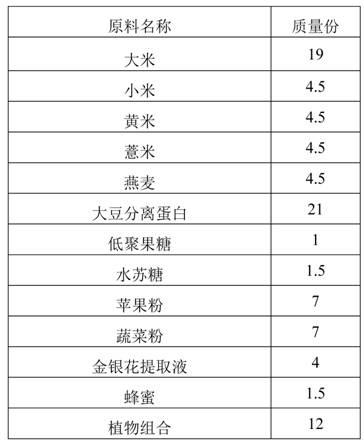
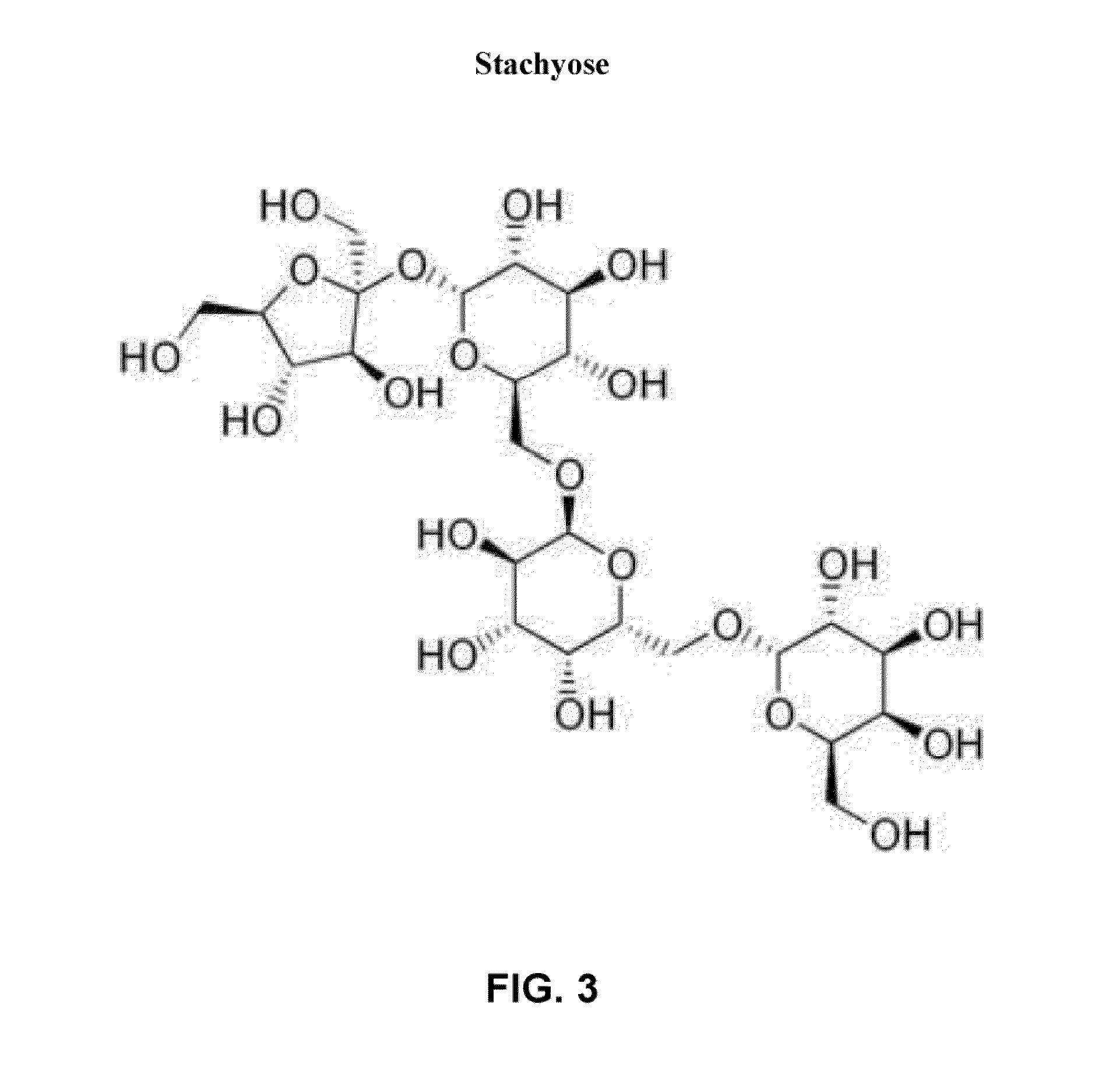
The invention discloses plant nutrient powder suitable for babies 12-24 months old. The plant nutrient powder comprises the following raw materials in parts by mass: 18-22 parts of rice, 4-6 parts of millet, 4-6 parts of glutinous millet, 4-6 parts of pearl barley, 4-6 parts of oat, 20-24 parts of isolated soy protein, 0.5-3.5 parts of fructooligosaccharide, 1.5-2.5 part of stachyose, 6-10 parts of apple powder, 6-10 parts of vegetable powder, 3-7 parts of honeysuckle extract, 1.5-2 parts of honey and 11-15 parts of plant. The plant nutrient powder disclosed by the invention is rich in various plant proteins, can prevent a baby from eating milk animal nutrition excessively, balance balanced diet, promote healthy growth of a baby and avoid allergy caused by animal proteins; moreover, since no lactose is in the formula of the plant nutrient powder, the baby can be prevented from having lactose intolerance.
Owner:广东和善衡营养品有限公司
High-protein milk and production method thereof
InactiveCN106804707AThe taste is fresh, smooth and delicateFull of nutritionMilk preparationOther dairy technologyLactasePasteurization
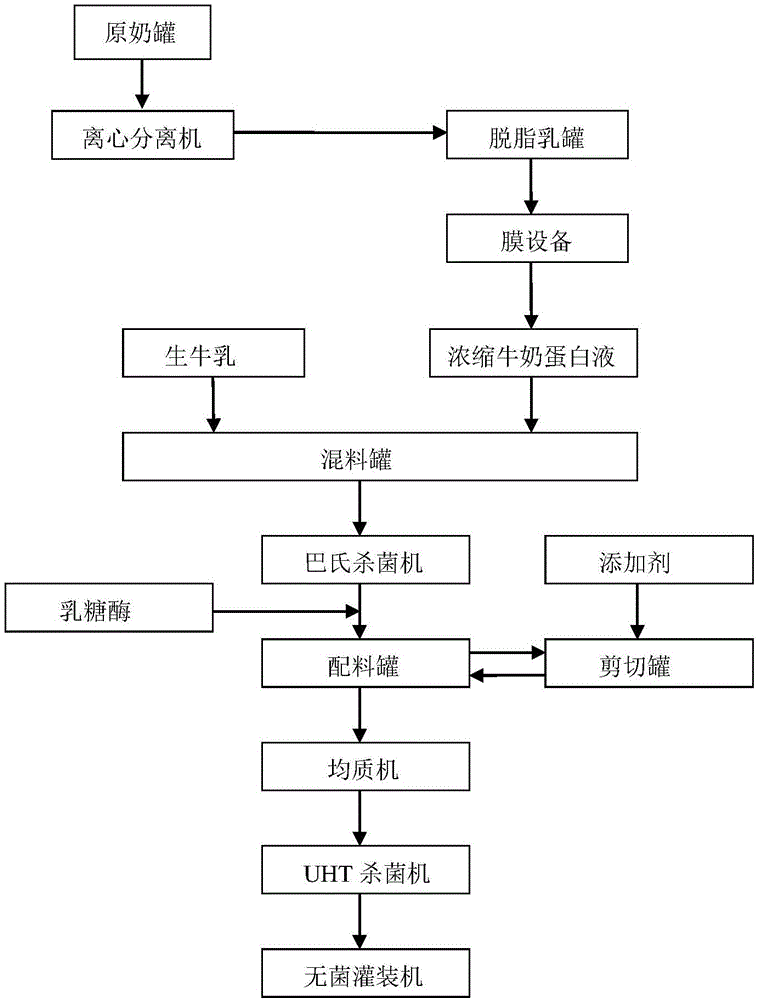
The invention relates to a production method of high-protein milk and belongs to the field of dairy product processing. The production method includes: using raw milk to produce concentrated milk protein liquid, evenly mixing the raw milk with the concentrated milk protein liquid, performing pasteurization and cooling, adding lactase for enzymolysis, weighing and evenly mixing white granulated sugar and additives, performing shear thinning on the material obtained after the enzymolysis and the mixture of the white granulated sugar and the additives, evenly mixing, homogenizing, sterilizing and performing sterile filling. By the production method, the high-protein milk can be produced by using the liquid protein, and the produced high-protein milk is fresh, fine and smooth in taste, rich in nutrition, applicable to lactose-intolerant people, low in fat content, low in calorie value and healthy.
Owner:宁夏塞尚乳业有限公司
Lactose-free infant formula food and preparation method thereof
InactiveCN109156817AAvoid intoleranceSmall particle sizeSugar food ingredientsVitamin food ingredientsLactose free infant formulaAdditive ingredient
The invention provides a lactose-free infant formula food and a preparation method thereof. The preparation method disclosed by the invention comprises the following steps: adopting a dry-wet compounding process, namely producing vegetable fat powder by a wet process, and producing lactose-free whole milk powder by a lactose digestion process; performing dry mixing on the vegetable fat powder, thelactose-free whole milk powder, other main ingredients and auxiliary materials and bioactive substances, thereby obtaining the formula food suitable for lactose intolerant infants at an age of 0-12 months. According to the formula, the lactose is completely replaced with other carbohydrates, the lactose content of the product is less than 0.5wt%, and 1.8-2.2wt% of galactose having an important effect on brain development of the infants is remained. The protein in the formula is provided by lactoprotein, and the formula food is suitable to be eaten by the lactose intolerant infants so as to avoid lactose intolerance.
Owner:贝因美(杭州)食品研究院有限公司 +1
Lactose-free infant formula milk powder and preparation method thereof
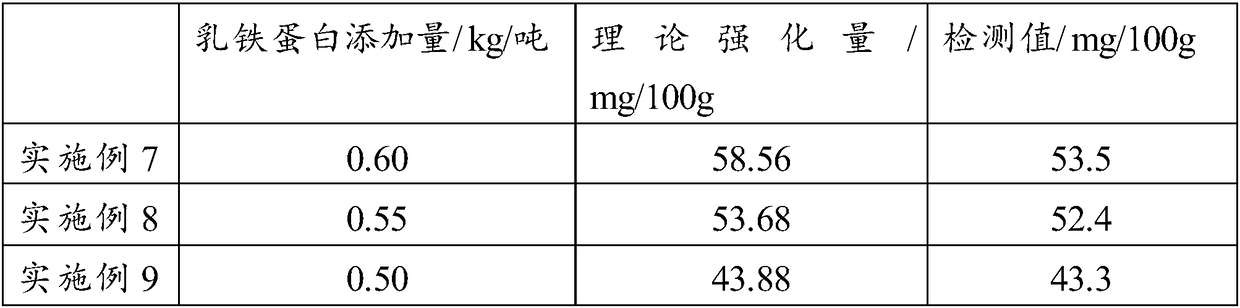
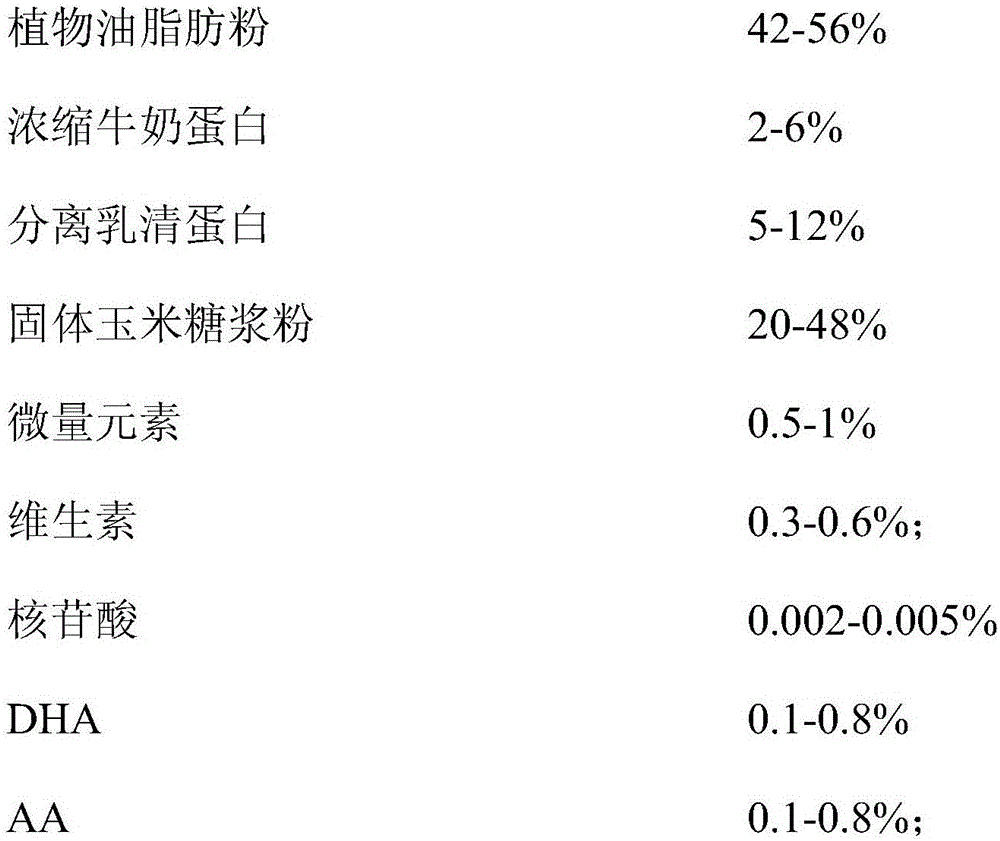
The invention relates to the technical field of food ingredients, specifically to a lactose-free infant formula milk powder for special medical use and a preparation method thereof. A special medical condition is primary or secondary lactose intolerant babies. The lactose-free infant formula milk powder mainly comprises the following ingredients (by weight): 35-70% of plant oil fatty powder, 1-10% of concentrated milk protein, 3-15% of separated whey protein, 20-50% of solid corn syrup powder, 0.1-5% of trace elements and 0.1-5% of vitamins, wherein sum of contents of the ingredients is no more than 100%, and content of lactose is less than 0.5g / 100g. The lactose-free infant formula milk powder contains 30 vitamins and trace elements which are essential to infants daily, linoleic acid, alpha-linolenic acid / aliphatic acid combination, DHA, AA and nucleotide, and lactose content meets requirements of GB25596-2010 on lactose content of lactose-free formula milk powder. In comparison with a common formula milk powder (containing lactose), the product meets health requirements of lactose intolerant lactose-free infants.
Owner:无锡超科食品有限公司
Lactose-intolerance fermented milk and preparation method thereof
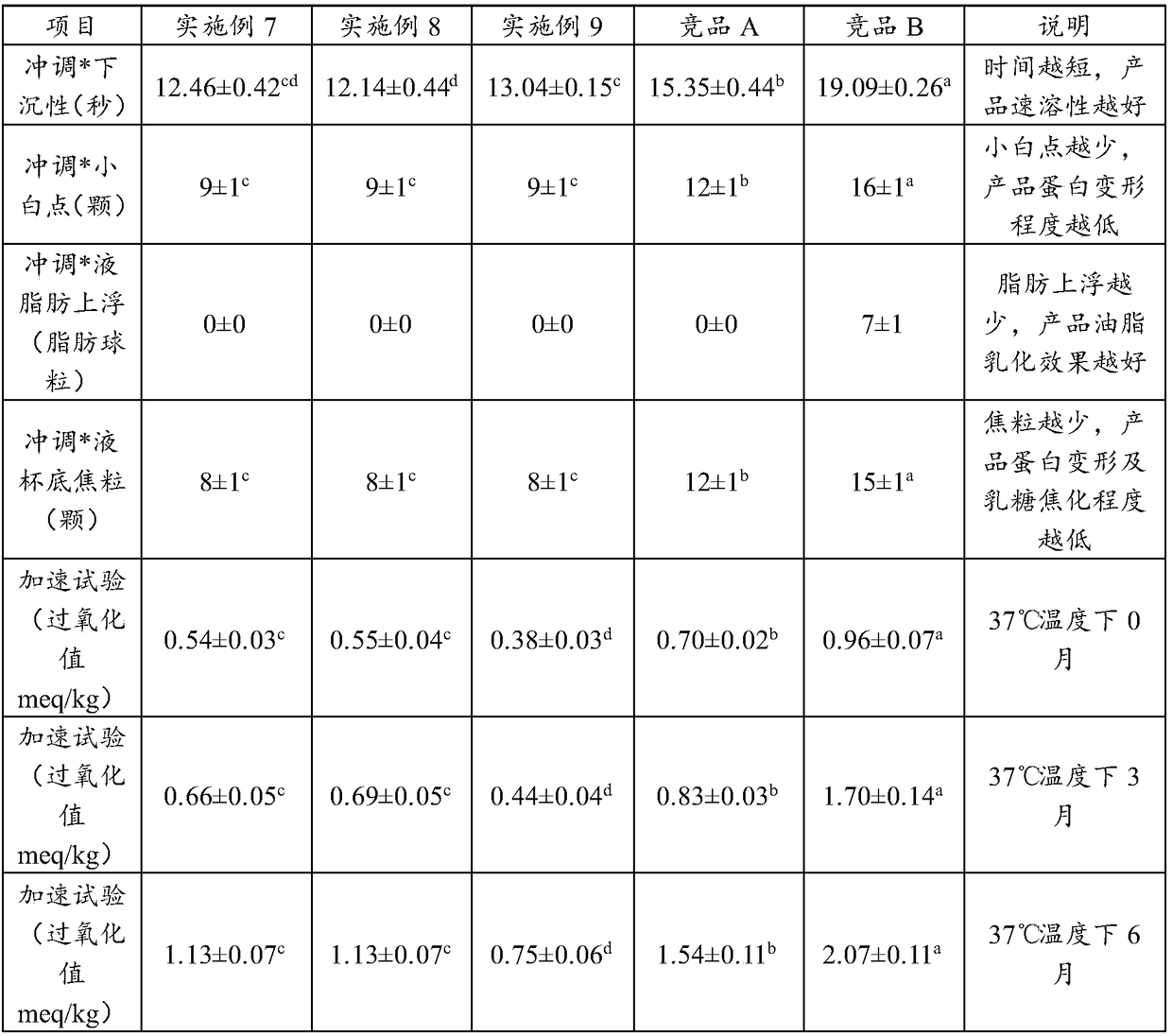
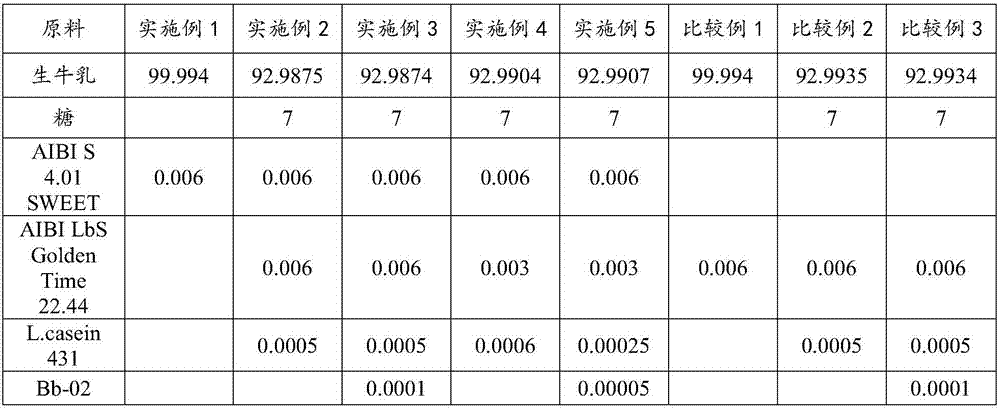
PendingCN107136214ARefreshing tasteDelicate tissue stateMilk preparationLactobacillusLactaseLactose intolerant
The invention provides lactose-intolerance fermented milk. The lactose-intolerance fermented milk is prepared by fermenting, by weight, 92.9874-99.994% of raw milk, 0-7% of sugar and 0.006-0.0126% of a fermentation agent; the fermentation agent comprises streptococcus thermophilus AIBI S 4.01 SWEET purchased from the Soyuz Nabob group. Lactase and a stabilizer are not added in the raw materials of the fermented milk, the lactose is degraded with special streptococcus thermophilus, and the fermented milk is refreshing in taste and delicate in tissue; the lactose content in the fermented milk is 0.014-0.5%, lactose-intolerance standard stipulated internationally is achieved, the problem about use of the fermented milk by lactose intolerant patients is solved, and the fermented milk in rich and full in nutrition and has the effect of immune disease prevention; during preparation, enzymolysis time of the lactase is omitted, no equipment and process is added, and production cycle of the fermented milk is short.
Owner:BRIGHT DAIRY & FOOD
Prebiotic Formulations and Methods of Use
The invention provides methods and pharmaceutical compositions for treating symptoms associated with lactose intolerance and for overall improvement in gastrointestinal health. Described herein are methods and pharmaceutical compositions for improving overall gastrointestinal health or for decreasing symptoms of lactose intolerance by administering to subject in need thereof a pharmaceutical composition comprising a prebiotic, optionally in combination with effective amount of a probiotic microbe or microbes. The invention also provides diagnostic instruments and methods of diagnosing lactose intolerance using the diagnostic instruments.
Owner:RITTER PHARMA
Self-contained, portable h2/co2 (AIR) ratio apparatus
InactiveUS20160143561A1Medical automated diagnosisRespiratory organ evaluationHydrogen concentrationLactose intolerance
A self-contained, portable H2 / CO2 (air) ratio apparatus has an exhaled breath system, an air analyzing chamber, a hydrogen concentration analyzer, a carbon dioxide (air) concentration analyzer, an air outlet, a computational analyzer and a display unit. The display unit can be a part of the self-contained, portable H2 / CO2 (air) ratio apparatus, remotely a part of the self-contained, portable H2 / CO2 (air) ratio apparatus, or combinations thereof. The apparatus can assist in the diagnosis of a neonatal patient developing necrotizing enterocolitis and / or lactose intolerance.
Owner:ONY
High-purity galactooligosaccharide compositions, preparations, and applications thereof
ActiveUS20170211112A1Simple manufacturing processLower blood sugar levelsOrganic active ingredientsMetabolism disorderBiotechnologyIntestinal microorganisms
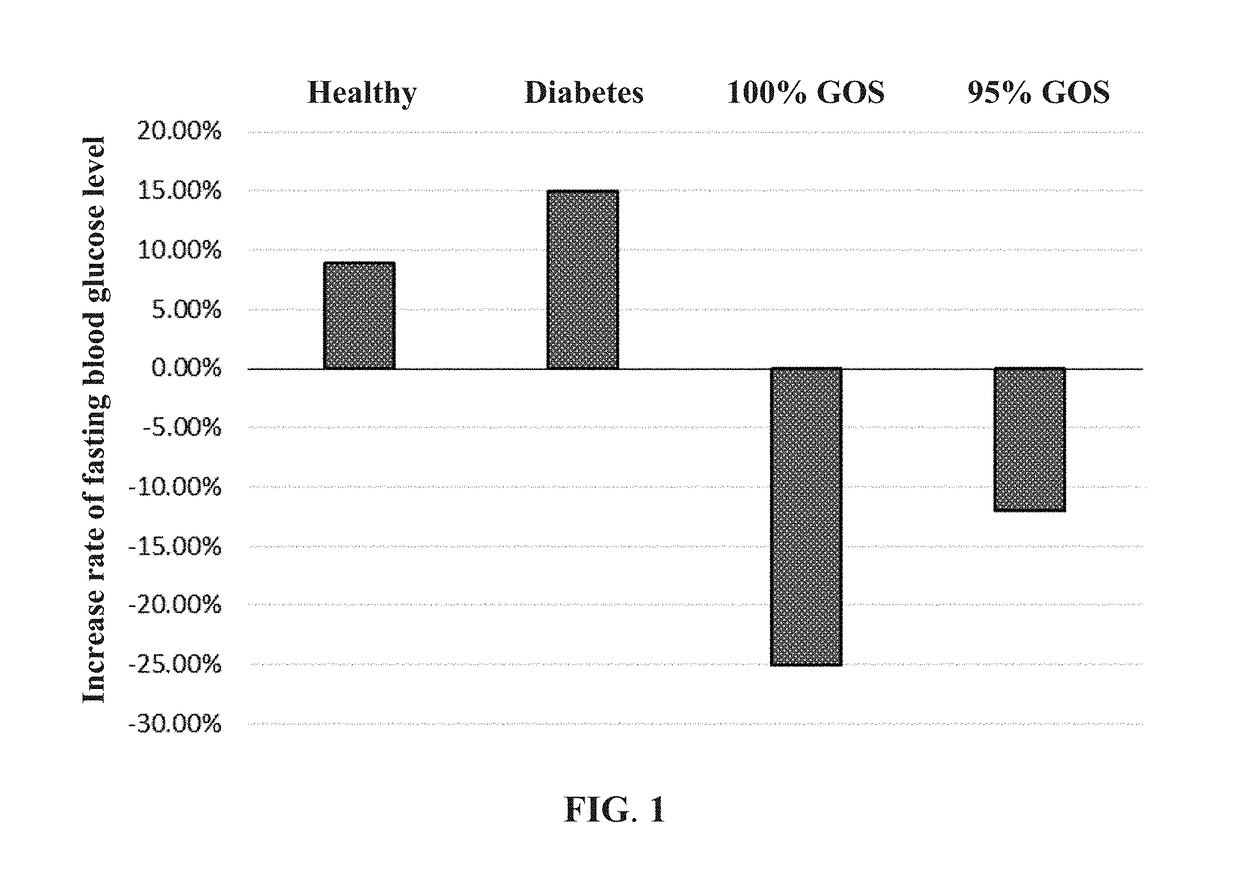
Provided is a method of preparing a high-purity galactooligosaccharide composition by fermentation of a low-purity galactooligosaccharide mixture with a yeast strain, Kluyveromyces lactis ATCC 8585. The high-purity galactooligosaccharide composition includes at least 99% of galactooligosaccharides selected from the group consisting of galactotriose, galactotetrose, galactooligosaccharides with five or more sugar units, and combinations thereof and lower than 1% of monosaccharides and disaccharides. Also provided is a high-purity galactooligosaccharide composition and applications thereof in regulating blood glucose level and improving gut microbiota. For example, the high-purity galactooligosaccharide composition may be used to manufacture food products, beverages, health food products, nutritional supplements, and pharmaceutical compositions for patients or pets afflicted with diabetes mellitus or lactose intolerance.
Owner:KING PREBIOTICS BIOTECH TW CO LTD
Lactose-free lipid-lowering milk and production method thereof
InactiveCN106070636AFit for consumptionSatisfactionMilk preparationOther dairy technologyLactose free milkAnimal science
The invention provides lactose-free lipid-lowering milk and a production method thereof. The lactose-free lipid-lowering milk is prepared from a main material and auxiliary materials in the weight ratio being (90-98):(2-10), wherein the main material is lactose-free milk, the auxiliary materials comprise 50-80 parts by weight of hawthorn fruit powder, 10-30 parts by weight of cassia seed powder, 10-20 parts by weight of black fungus polysaccharides and 0.1-1 part by weight of phytosterol. During preparation, firstly, components of the auxiliary materials are mixed in proportion and then mixed with the lactose-free milk, the mixture is homogenized and sterilized, and the lactose-free lipid-lowering milk is obtained. According to the lactose-free lipid-lowering milk, various Chinese herbal medicine components perform a synergistic function and have a remarkable curative effect on hyperlipidemia; the content of lactose in the lactose-free milk is lower than 0.1%, and the lactose-free lipid-lowering milk is suitable for lactose-intolerant people; due to complete removal of lactose and monosaccharide, the lactose-free lipid-lowering milk is also suitable for people who have higher requirements for the content of sugar, such as people suffering from diabetes.
Owner:方雅悯 +1
Healthy food compositions having gel or foam textures and comprising hydrolyzed egg products
ActiveUS20150173394A1Increase contentLow in fatConfectionerySweetmeatsAdditive ingredientHydrolysate
The invention relates to healthy food compositions with a gel or foam texture that have antioxidant properties, are rich in proteins, low-fat, lactose- and casein-free and easy-to-chew, comprising a neutral-flavored egg hydrolysate as a basic ingredient.These compositions are presented as an alternative to sweet and salty dishes using dairy products as a base because they have similar flavor, appearance and texture characteristics. These compositions are also presented as new foods that are particularly indicated for being consumed by people who are lactose intolerant or who have obesity issues.
Owner:CONSEJO SUPERIOR DE INVESTIGACIONES CIENTIFICAS (CSIC) +1
Hmo mixtures
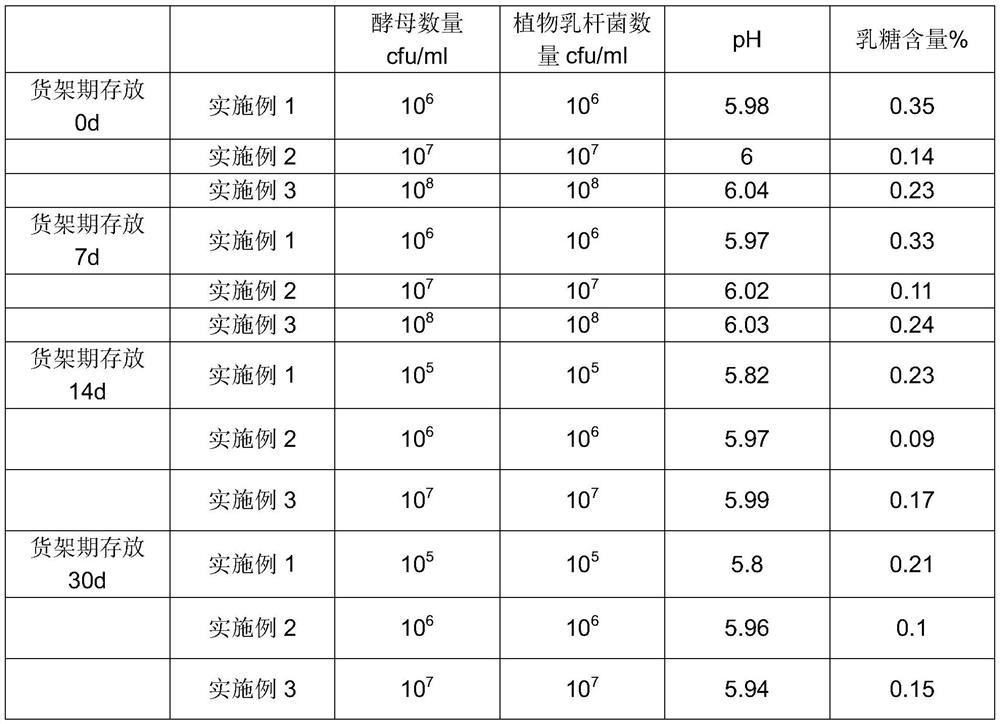
The present invention relates to human milk oligosaccharides (HMOs) for use in the following, synthetic compositions comprising HMO for use in the following, and methods for prophylactically reducing the severity and / or incidence of gastrointestinal IBS symptoms in lactose intolerant IBS patients; reintroducing a lactose source into the diet of lactose intolerant IBS patients while prophylactically reducing the gastrointestinal IBS symptom severity and / or symptom incidence in IBS patients; and / or reducing the severity and / or incidence of non-gastrointestinal symptoms in lactose intolerant IBS patients.
Owner:GLYCOM AS
Walnut calcium supplement effervescing preparation
The invention relates to a walnut calcium supplement effervescing preparation which is prepared from the following raw materials in parts by weight: 20-35 parts of walnut granules, 20-24 parts of ascorbic acid, 15-20 parts of calcium fumarate, 15-20 parts of calcium lactate and 15-20 parts of calcium gluconate and the like. The calcium supplement effervescing preparation provided by the invention contains multiple organic calcium and inorganic calcium and is more beneficial to absorption of calcium of human body, reduction of morbidity of osteoporosis and maintenance of hardness of skeleton and tooth, and the calcium supplement effervescing preparation can nourish brain. The calcium supplement effervescing preparation provided by the invention contains lactase and is especially applicable to people who suffer from lactose intolerance.
Owner:CHANGSHU HUIFENG FOOD
Lactose-free micro-fermented cow milk and preparation method thereof
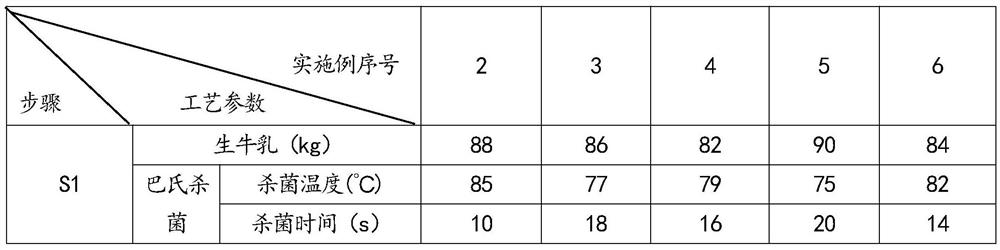
The invention discloses lactose-free micro-fermented cow milk and a preparation method thereof. The micro-fermented cow milk is prepared from the following raw materials in percentage by mass: 99.925 to 99.971 percent of raw milk, 0.008 to 0.012 percent of yeast, 0.001 to 0.003 percent of lactobacillus plantarum and 0.02 to 0.06 percent of lactase. The preparation method of the lactose-free micro-fermented cow milk comprises the following steps: S1, preheating raw milk, and degreasing to obtain skimmed milk and single cream; S2, sterilizing the skimmed milk in S1, adding yeast and lactase, heating, adding lactobacillus plantarum, and carrying out fermentation hydrolysis to obtain a fermentation hydrolysis feed liquid; and S3, sterilizing the single cream in the S1, adding the single cream into the fermentation hydrolysis feed liquid in the S2, preheating, carrying out sterile homogenization, cooling and filling to obtain the product. The micro-fermented milk disclosed by the invention does not contain lactose, is suitable for lactose intolerance consumer groups, and is good in flavor and taste.
Owner:BRIGHT DAIRY & FOOD
Lactase sheet and preparing method thereof
The invention discloses a tablet formulation against lactose intolerance and alactasia and the preparation method. The formulation includes active ingredient lactase, excipient diluent agent, disintegrating agent, binder, wetting agent, glidant and lubricant. Among starch, microcrystalline cellulose, mannitol and pre-gelatinized starch, one ingredient or more than two ingredient compound can be chosen as diluent agent (filling agent); one or more ingredients can be chosen from carboxymethyl starch, crosslinking sodium carboxymethyl cellulose, crosslinking povidone, low-substituted hydroxypropyl cellulose, microcrystalline cellulose and pre-gelatinized starch as disintegrating agent; one ingredient can be chosen from povidone, starch pulp and any cellulose as binder; one ingredient can be chosen from micro-powder silica gel and talcum powder to be used as glidant; one ingredient can be chosen from magnesium stearate, calcium stearate, stearic acid, hydrogenated vegetable oil and carmowax as lubricant; one ingredient can be chosen from ethanol, water and ethanol water as wetting agent.
Owner:郭炳华 +2
Zero-lactose modified milk browned by Maillard reaction and preparation method thereof
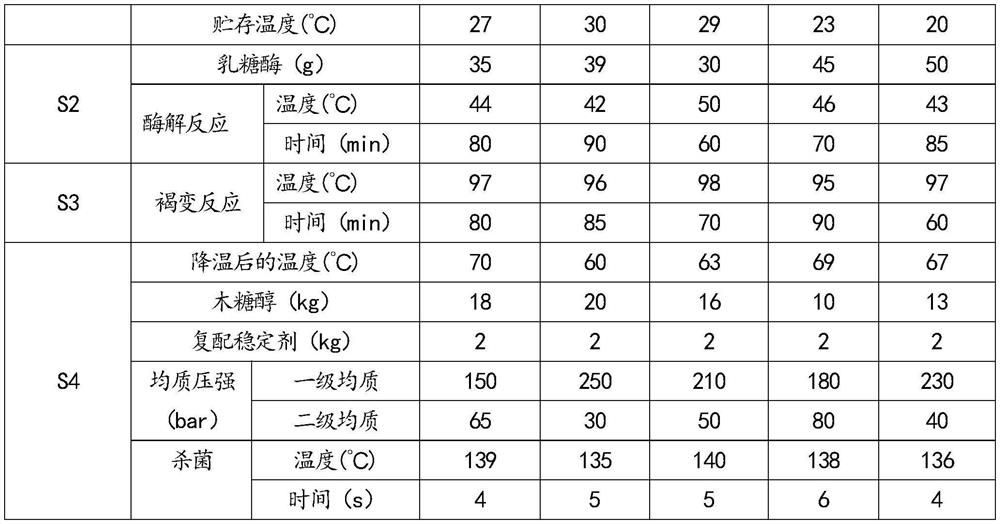
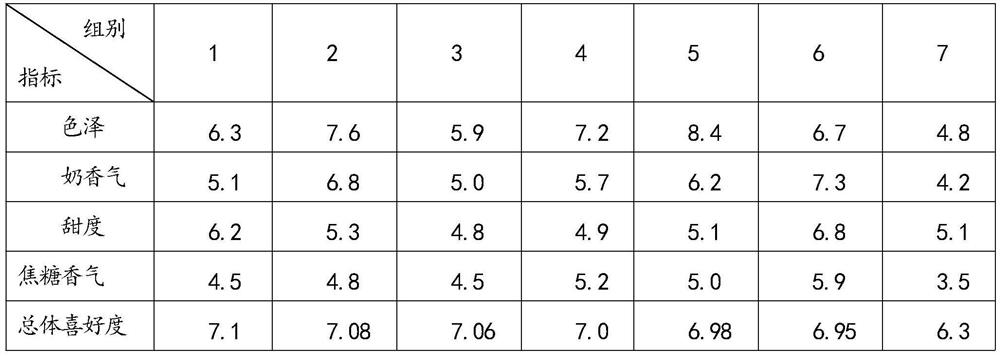
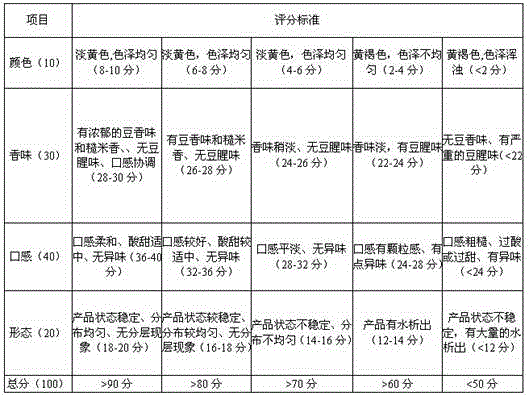
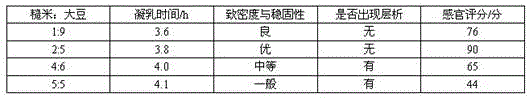
PendingCN113396973ASufficient energyHigh texture stabilityMilk preparationBiotechnologyMaillard reaction
Owner:JUNLEBAO DAIRY GRP CO LTD
Deep hydrolyzed protein formula powder and preparation method thereof
PendingCN110037114ARelieve diarrheaLow recurrence rateMilk substitutesDocosahexaenoic acidMilk protein
The invention aims at disclosing deep hydrolyzed protein formula powder and a preparation method thereof. The deep hydrolyzed protein formula powder is prepared from the following components in mass percent: 70-80% of vegetable fat powder, 10-20% of deep hydrolyzed protein powder, 1-5% of glucose syrup dry powder, 1-5% of a nutritional enhancer, 1-5% of fructo-oligosaccharide, 1-3% of arachidonicacid powder and 1-3% of docosahexaenoic acid powder. Compared with the prior art, the formula powder is reasonable in formulation, relieves complications such as infantile diarrhea, eczema and asthmacaused by milk protein allergy, and solves the problem of infantile diarrhea while solving protein allergy. For lactose intolerant people, the formula powder can be better digested and absorbed, and alleviate the problem of diarrhea in allergic people, achieving dual purposes. Addition of the nutritional enhancer ensures comprehensive and reasonable nutrition in the development stage of newborns,and meets the normal needs of physiology and life, thereby maintaining and improving the health level of the newborns. The formula powder is reasonable in design, easy to produce and convenient to control, and realizes the purposes of the formula powder.
Owner:浙江科露宝食品有限公司
Composition for use in the therapy of lactose intolerance or conditions arising from lactase deficiency
The invention provides a composition for use in therapy of lactose intolerance or conditions arising from lactase deficiency, wherein the composition is a non-dairy solid dosage form comprising:(a) a lactase; and(b) one or more lactase-producing and / or lactase containing microorganisms selected from Lactobacillus delbrueckii ssp bulgaricus and Streptococcus thermophilus; wherein the lactase (a) is other than a lactase derived from the said one or more microorganisms (b); and wherein the one or more lactase-producing and / or lactase-containing microorganisms (b) are in dried form.Also provided are uncoated capsule and tablet compositions containing the lactase and the lactase-producing and / or lactase-containing microorganisms.
Owner:VITACARE
A kind of preparation method of stirring brown rice fermented drink
A method for preparing a stirred fermented brown rice beverage, inoculating the compound bacteria powder mixed with Lactobacillus plantarum, Lactobacillus acidophilus, Lactobacillus rhamnosus and Lactobacillus casei on a potato medium to obtain a compound culture solution; Take puffed brown rice flour, add pure water and stir into a uniform paste, heat in a water bath, adjust the pH value, add α-amylase for enzymatic hydrolysis reaction; boil for sterilization, deenzyme treatment, filter to remove filter residue; Add pure water to soak, add the filtered brown rice paste and add pure water to make up the volume, stir evenly, carry out ultrafine grinding, add sucrose, carry out pasteurization, then insert the compound culture medium, stir evenly, ferment , to obtain a stirred fermented brown rice drink. The advantages are: rich nutrition, delicate taste, probiotics, can improve lactose intolerance, promote gastrointestinal motility, reduce the anti-nutritional factor phytic acid in brown rice, and avoid the symptoms of flatulence and discomfort caused by eating a lot of beans.
Owner:BENXI ZHAIXIANG ECOLOGICAL AGRI
A kind of preparation method of lotus root yogurt
ActiveCN107372823BReduce bitternessPreserve health benefitsMilk preparationBiotechnologyGastrointestinal cancer
The invention provides a preparation method of lotus root yoghourt, relating to the technical field of food processing. The preparation method comprises the following steps: (1) preparing lotus root juice; (2) preparing yoghourt raw materials; and (3) preparing the lotus root yoghourt. By utilizing a specific formula and a secret preparation process, most nutrients of two foods, namely the lotus root and the yoghourt, can be guaranteed, the combined effect of the lotus root and the yoghourt can be realized, dietary fibers in the lotus root are capable of preventing gastrointestinal cancer, the yoghourt is capable of preventing lactose intolerance, and the medical value of the lotus root and the health effects of the yoghourt are combined together; and the lotus root yoghourt is a healthy beverage suitable for people of all ages.
Owner:BAISE UNIV
Healthy food compositions having gel or foam textures and comprising hydrolyzed egg products
ActiveUS9648897B2Delaying the oxidation process involved in consumer body deteriorationReducing food deteriorationProtein composition from eggsConfectioneryBiotechnologyAnimal science
Owner:CONSEJO SUPERIOR DE INVESTIGACIONES CIENTIFICAS (CSIC) +1
Strawberry clavulanic acid milk beverage and preparation method thereof
The invention provides a strawberry clavulanic acid milk beverage which comprises the following raw materials in parts by weight: 300-500 parts of raw goat milk, 25-40 parts of white granulated sugar, 0.04-1 part of acesulfame potassium, 5-9 parts of strawberry juice, 0.1-1 part of sodium citrate, 1-6 parts of lactic acid, 1.2-4 parts of citric acid, 0.5-7 parts of soluble dietary fiber, 0.01-0.08 part of salt, 0.05-1 part of broken pine pollen, 1.5-6 parts of stabilizer, 0.1-5 parts of sodium cyclamate, 0.1-0.6 part of potassium sorbate and 0.05-0.55 part of edible essence. The invention also provides a preparation method for the strawberry clavulanic acid milk beverage. The preparation method comprises the steps of reducing pressure, deodorizing and homogenizing. The strawberry clavulanic acid milk beverage prepared according to the method provided by the invention is low in lactose content, is capable of relieving the untoward effect to the lactose intolerant patient and meets the requirement of the lactose intolerant patient. The lactose content is 0.01-0.02% and is lower than that of the marketing milk beverage.
Owner:山东坤泰生物科技有限公司
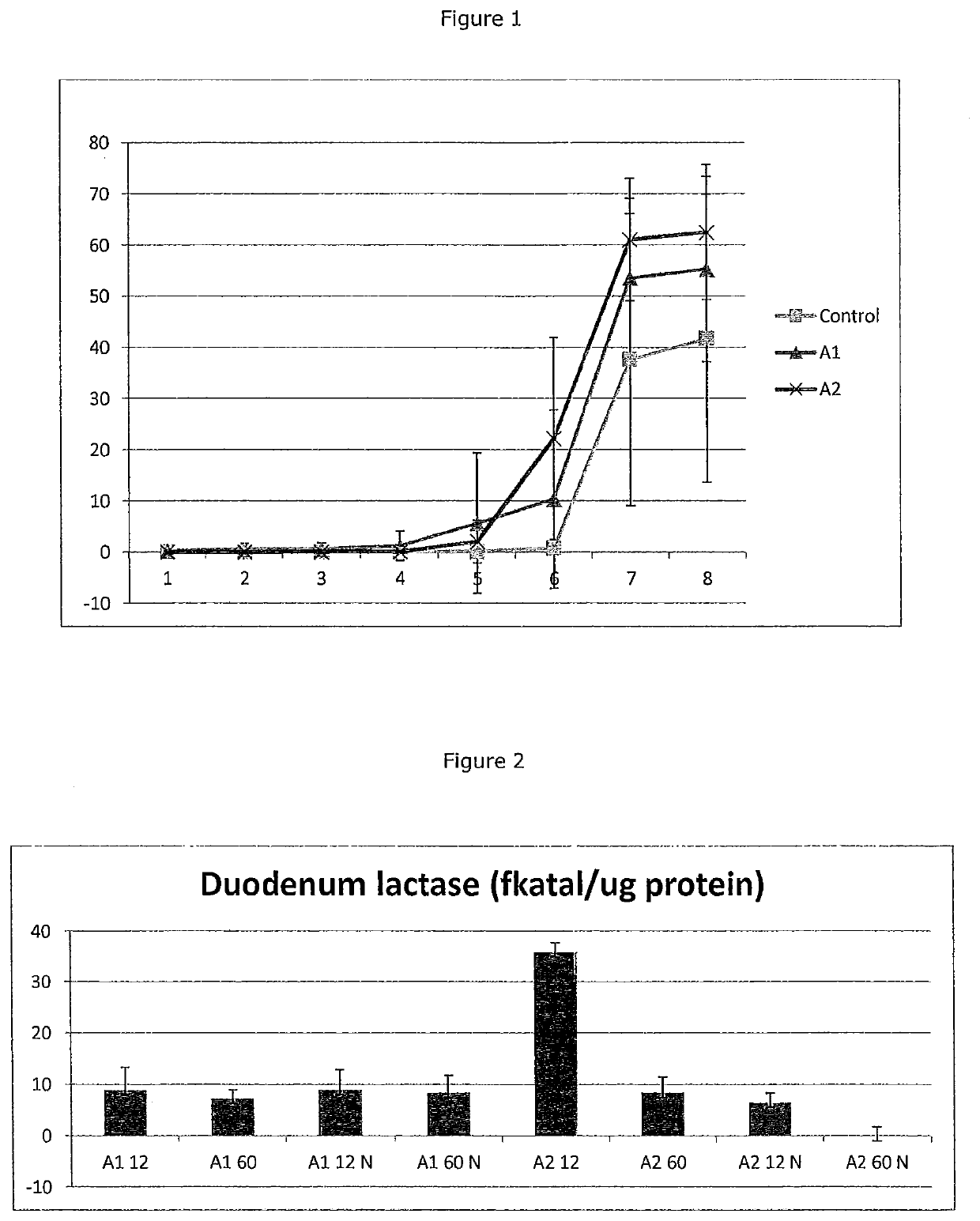
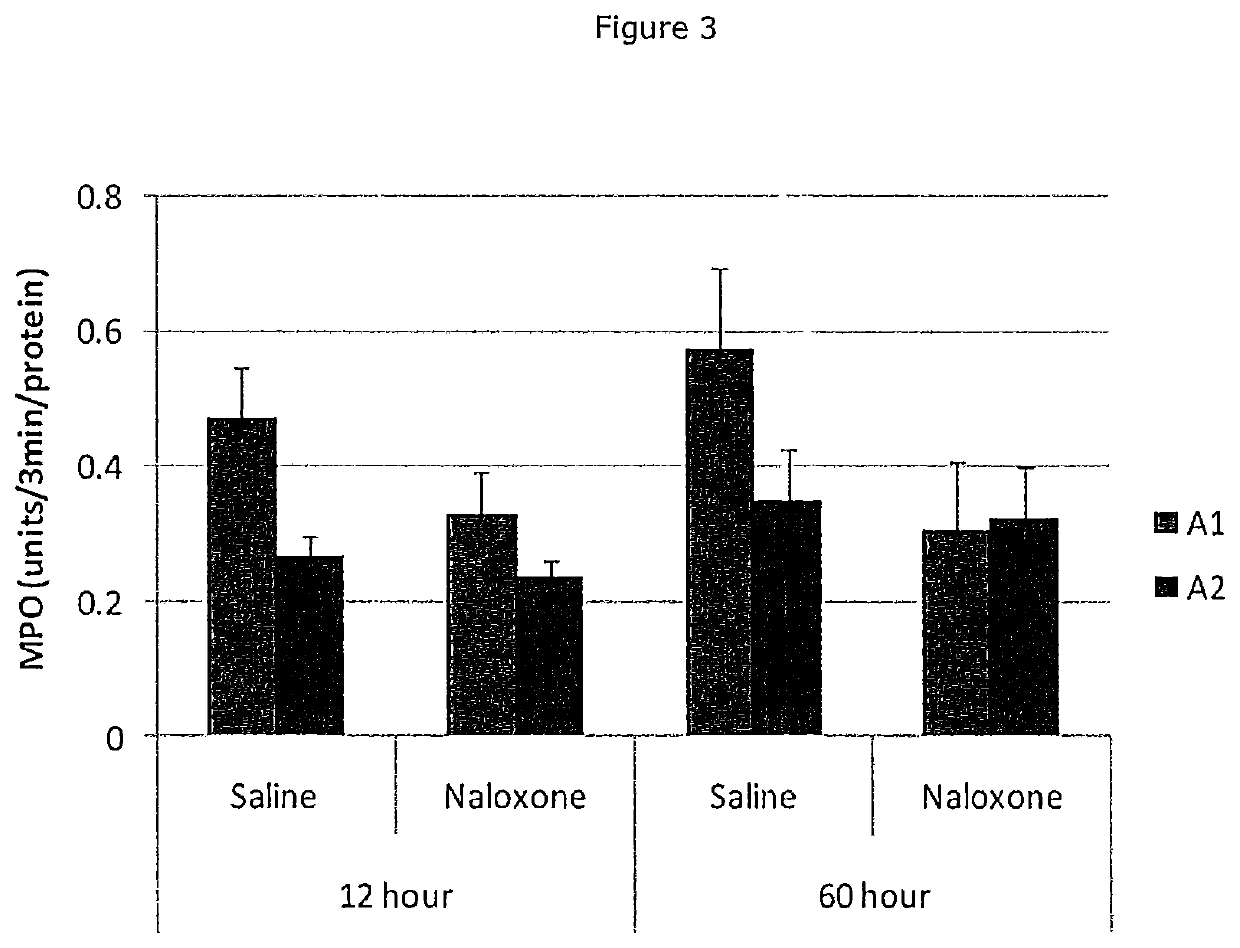
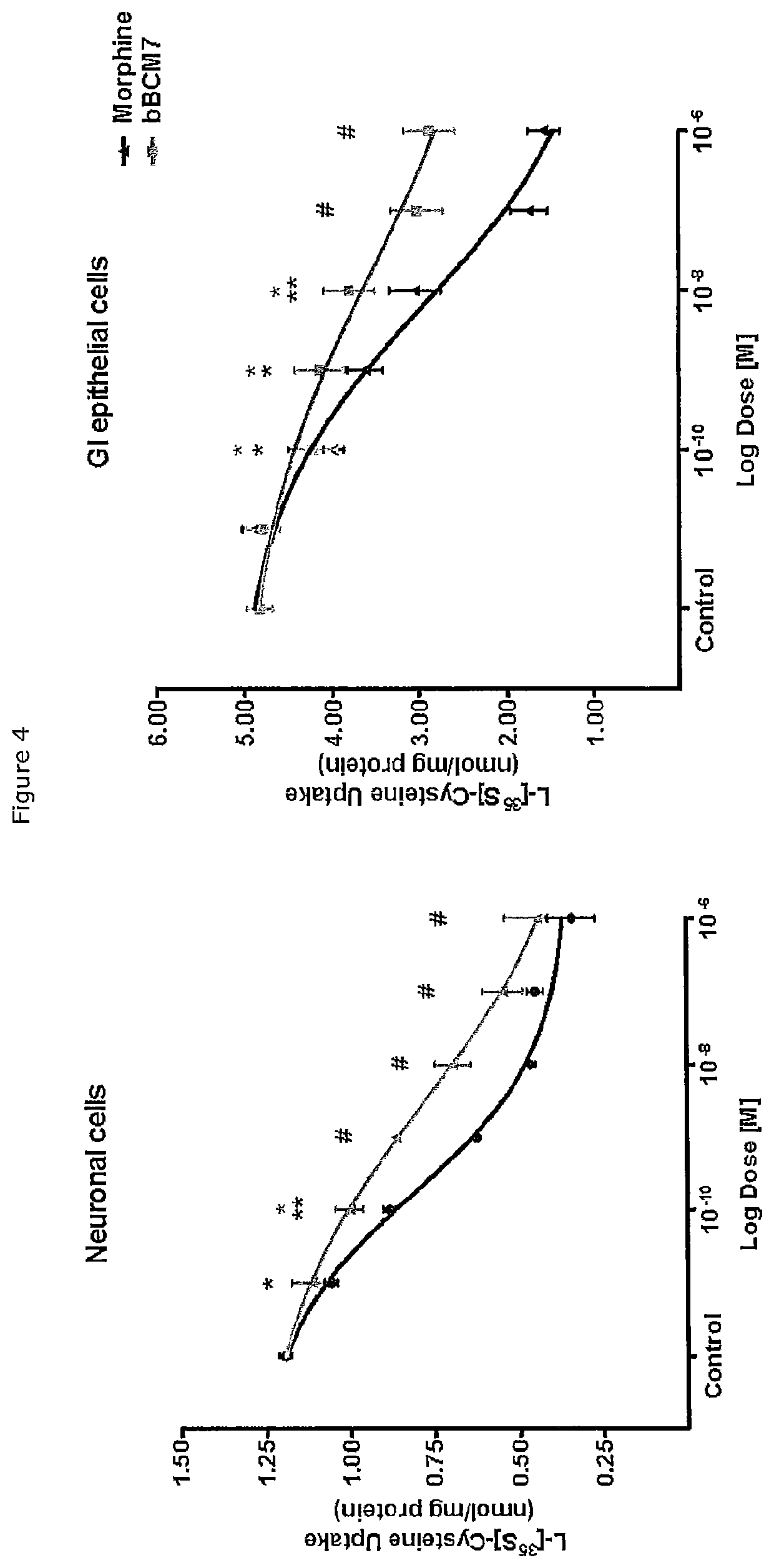
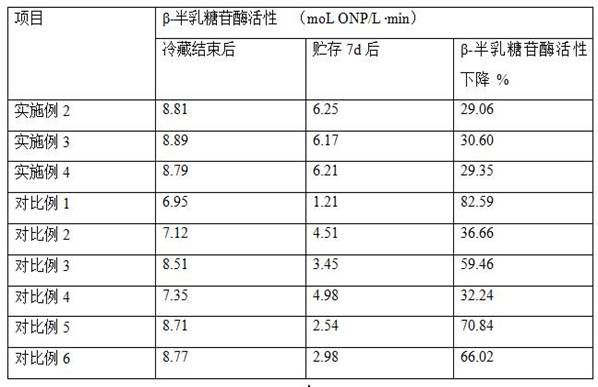
Beta-casein a2 and reducing or preventing symptoms of lactose intolerance
InactiveUS20200147173A1Reducing and preventing symptomDispersion deliveryPeptide/protein ingredientsPhysiologyLactose intolerant
Owner:A2 MILK CO LTD
Probiotic fermented milk suitable for people suffering from lactose intolerance and preparation method of probiotic fermented milk
The invention discloses probiotic fermented milk suitable for people suffering from lactose intolerance and a preparation method of the probiotic fermented milk, and belongs to the field of health-care foods. The preparation method comprises the following steps: cleaning and drying jerusalem artichoke stalks, crushing and sieving to obtain jerusalem artichoke stalk particles, heating and extracting the jerusalem artichoke stalk particles with distilled water, filtering, and concentrating to obtain an extract; carrying out silica gel column chromatography on the extract, eluting with ethyl acetate, collecting eluent, and carrying out rotary evaporation to obtain a jerusalem artichoke stem extract; mixing milk with the jerusalem artichoke stem extract, sterilizing, and adding a probiotic leavening agent for fermentation to obtain yoghourt after fermentation is finished; and adding inulin and fructo-oligosaccharide into the yoghourt, uniformly mixing, refrigerating, and performing after-ripening to obtain the probiotic fermented milk suitable for people suffering from lactose intolerance. The jerusalem artichoke is treated and fermented together with the milk, and then the inulin and the fructo-oligosaccharide are added, so that the activity of beta-galactosidase generated by probiotics is greatly improved, and adverse symptoms generated when L1 patients take milk products are remarkably relieved.
Owner:山东福乐维康生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com