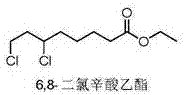
6,8-dichloro ethyl caprylate preparation method
A technology for ethyl dichlorooctanoate and ethyl chlorooctanoate is applied in the field of preparation of ethyl 6,8-dichlorooctanoate, and can solve the problems of intractable industrial waste water, strong corrosiveness, inability to be recovered by distillation and other methods, and the like, It is suitable for large-scale industrial production, the reaction conditions are mild and easy to control, and the product yield and product purity are high.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Under ice-water bath conditions, put 297Kg thionyl chloride and 1485Kg toluene into a 3000L reactor, slowly add 540Kg 6-hydroxy-8-chlorooctanoic acid ethyl ester dropwise, and at the same time pass dry air into it to continue bubbling, and control the air flow rate to 6.48 L / min (that is, the air flow rate is controlled at 0.012L / min per kilogram of 6-hydroxy-8-chlorooctanoic acid ethyl ester), after 2 hours, the temperature is raised to 30-35°C, kept for 1 hour, and then heated to about 90 ~100°C, stop bubbling, keep warm for 2 hours, the reaction solution is neutralized to neutral with 20% sodium carbonate aqueous solution, separate layers, the organic layer is evaporated to remove the solvent under normal pressure, and the 170~180 ℃ distillate to obtain 480Kg 6,8-dichlorooctanoic acid ethyl ester with a purity of 98.3%.
Embodiment 2
[0022] Under ice-water bath conditions, put 324Kg thionyl chloride and 1300Kg toluene into a 3000L reactor, slowly add 540Kg 6-hydroxy-8-chlorooctanoic acid ethyl ester dropwise, and simultaneously feed dry nitrogen to continue bubbling, and control the nitrogen flow rate to 8.1 L / min (that is, the nitrogen flow rate of 6-hydroxy-8-chlorooctanoic acid ethyl ester is controlled at 0.015L / min), after 2 hours, the temperature is raised to 30-35°C, kept for 1 hour, and then heated to about 90 ~100°C, stop bubbling, keep warm for 2 hours, the reaction solution is neutralized to neutral with 20% sodium carbonate aqueous solution, separate layers, the organic layer is evaporated to remove the solvent under normal pressure, and the 170~180 ℃ distillate to obtain 490Kg 6,8-dichlorooctanoic acid ethyl ester with a purity of 98.5%.
Embodiment 3
[0024] Under ice-water bath condition, drop 350Kg thionyl chloride and 1050Kg toluene into 3000L reaction kettle, slowly add dropwise 540Kg6-hydroxyl-8-chlorooctanoic acid ethyl ester, pass into dry air at the same time and continue bubbling, drop in 2 hours, and Control the air flow rate to 8.1L / min (that is, control the air flow rate at 0.015L / min per kilogram of 6-hydroxy-8-chlorooctanoic acid ethyl ester), then raise the temperature to 30-35°C, keep it warm for 1 hour, and then raise the temperature to about 90 ~100°C, stop bubbling, keep warm for 2 hours, the reaction solution is neutralized to neutral with 20% sodium carbonate aqueous solution, separate layers, the organic layer is evaporated to remove the solvent under normal pressure, and the 170~180 ℃ distillate to obtain 485Kg of 6,8-dichlorooctanoic acid ethyl ester with a purity of 98.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com