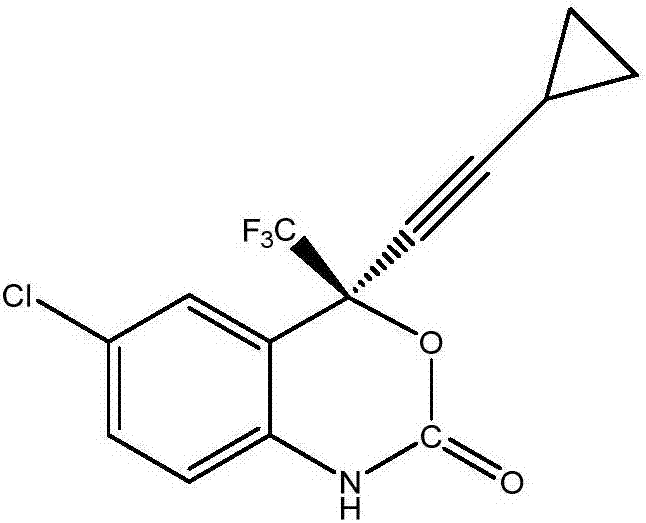
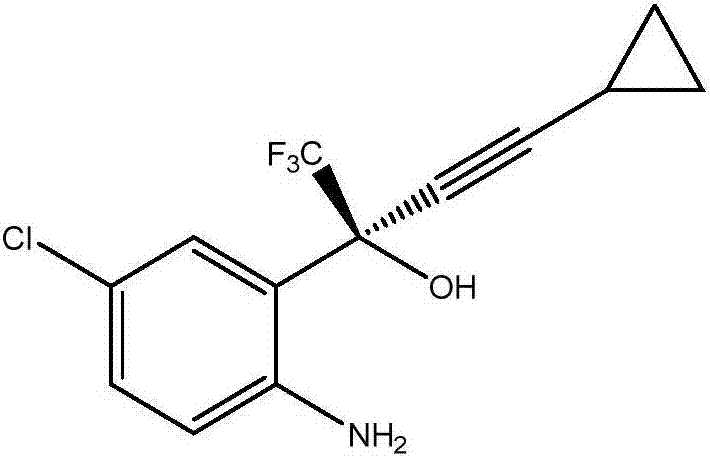
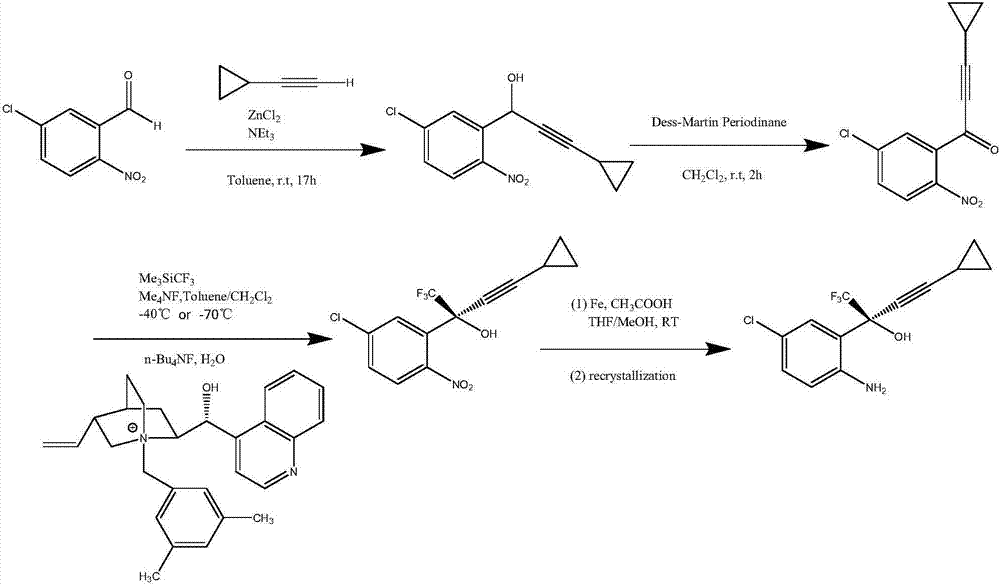
Preparation method of efavirenz intermediate
A technology of efavirenz and intermediates, which is applied in the field of organic synthesis, can solve the problems of complex organic ligand structure, cumbersome operation, and harsh reaction conditions, and achieve the advantages of recycling, shortening the process flow, and mild process conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] At 12°C, 52.36g of organic ligand (1R,2S)-1-phenyl-2-(1 -Toluene solution of -pyrrolidinyl)-1-propanol and 74.2g of zinc trifluoromethanesulfonate were stirred and reacted at this temperature for 2 hours. After the reaction was completed, the temperature was raised to 18°C. Then, a mixture of 25.47g cyclopropynemagnesium chloride and 80mL 2-methyltetrahydrofuran was added to the above solution, stirred at this temperature for 1 hour, then cooled to -10°C, and 43.11g 1-(5-chloro-2- Nitrophenyl)-2,2,2-trifluoroethanone, and the mixture was stirred at -10°C for 20 hours. After the reaction was completed, 400 mL of saturated ammonium chloride solution was added to quench the reaction, and the organic phase and the aqueous phase were separated by toluene extraction. The organic phase was directly concentrated and dried to obtain 83mL of the oil phase of (S)-1-(5-chloro-2-nitrophenyl)-1-trifluoromethyl-3-cyclopropyl-2-propyn-1-ol , it was added to 1500mL of tetrahydrofuran ...
Embodiment 2
[0029] At 12°C, 35.04g of organic ligand (1R,2S)-1-phenyl-2-(1 -Toluene solution of -pyrrolidinyl)-1-propanol and 49.48g of zinc trifluoromethanesulfonate were stirred and reacted at this temperature for 2 hours. After the reaction was completed, the temperature was raised to 18°C. Then add a mixture of 19.18g cyclopropynylmagnesium bromide and 70mL 2-methyltetrahydrofuran to the above solution, stir at this temperature for 1 hour, then cool down to -10°C, add 1-(5-chloro-2- Nitrophenyl)-2,2,2-trifluoroethanone (28.74 g), and the mixture was stirred at -10°C for 22 hours. After the reaction was completed, 300 mL of saturated ammonium chloride solution was added to quench the reaction, and the organic phase and the aqueous phase were separated by toluene extraction. The organic phase was directly concentrated and dried to obtain 78 mL of (S)-1-(5-chloro-2-nitrophenyl)-1-trifluoromethyl-3-cyclopropyl-2-propyn-1-ol as an oil phase, it was added to 1000mL of tetrahydrofuran and ...
Embodiment 3
[0031]At 12°C, add 30.12g organic ligand (1R,2S)-1-phenyl-2-(1 The toluene solution of -pyrrolidinyl)-1-propanol and 42.34g of zinc trifluoromethanesulfonate were stirred and reacted at 23°C for 2 hours. After the reaction was completed, the temperature was raised to 18°C. Then, a mixed solution of 18.38g cyclopropynemagnesium chloride and 80g 2-methyltetrahydrofuran was added to the above solution, stirred at this temperature for 1 hour, then cooled to -10°C, and 1-(5-chloro-2-nitro 23.95 g of phenyl)-2,2,2-trifluoroethanone, and the mixture was stirred at -6°C for 22 hours. After the reaction was completed, 260 mL of saturated ammonium chloride solution was added to quench the reaction, and the organic phase and the aqueous phase were separated by toluene extraction. The organic phase was directly concentrated and dried to obtain an oil phase of 71 mL of (S)-1-(5-chloro-2-nitrophenyl)-1-trifluoromethyl-3-cyclopropyl-2-propyn-1-ol , add it to 800mL of tetrahydrofuran and me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com