P-oxanone and L-phenylalanine copolymer and application thereof
A technology of phenylalanine and copolymer, which is applied in the field of p-oxycyclohexanone and L-phenylalanine copolymer and its application, and can solve problems such as poor mechanical strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Preparation and Characterization of PDPA Polymer
[0046] Table 1. PDPA series polymers
[0047]
[0048] a GPC method to determine its weight average molecular weight
[0049] Characterization of PDPA polymer
[0050] The L-phenylalanine content of PDPA polymerized by NMR 1 HNMR determination, specifically 1 For HNMR, see literature (J.APPL.POLYM.SCI.2013, DOI: 10.1002 / APP.39455).
[0051] Polymer molecular weight is determined by GPC method (DMF mobile phase), and the results are shown in Table 1.
Embodiment 2
[0053] Preparation method of each implant material
[0054] 1) Preparation method of medicine for preventing and treating scar hyperplasia and abdominal wall fibrosis
[0055] After the PDPA-5 polymer was synthesized, it was dissolved in chloroform, stirred and filtered to remove insoluble matter, and the obtained filtrate was slowly dropped into ether while stirring, and the polymer powder was precipitated, and the residual organic solvent was removed by vacuum drying for 24 hours. The obtained dry polymer powder is the implant drug used.
[0056] 2) Preparation method of composite film for preventing postoperative abdominal adhesion
[0057] Dissolve PDPA and PLGA in hexafluoroisopropanol according to the corresponding mass ratio to prepare a concentrated solution with a concentration of 10% w / v, and perform electrospinning to prepare a porous fiber membrane. The preparation parameters are as follows: injection rate 1mL / h , Voltage 8kv, ambient temperature 30°C, ambient re...
Embodiment 3
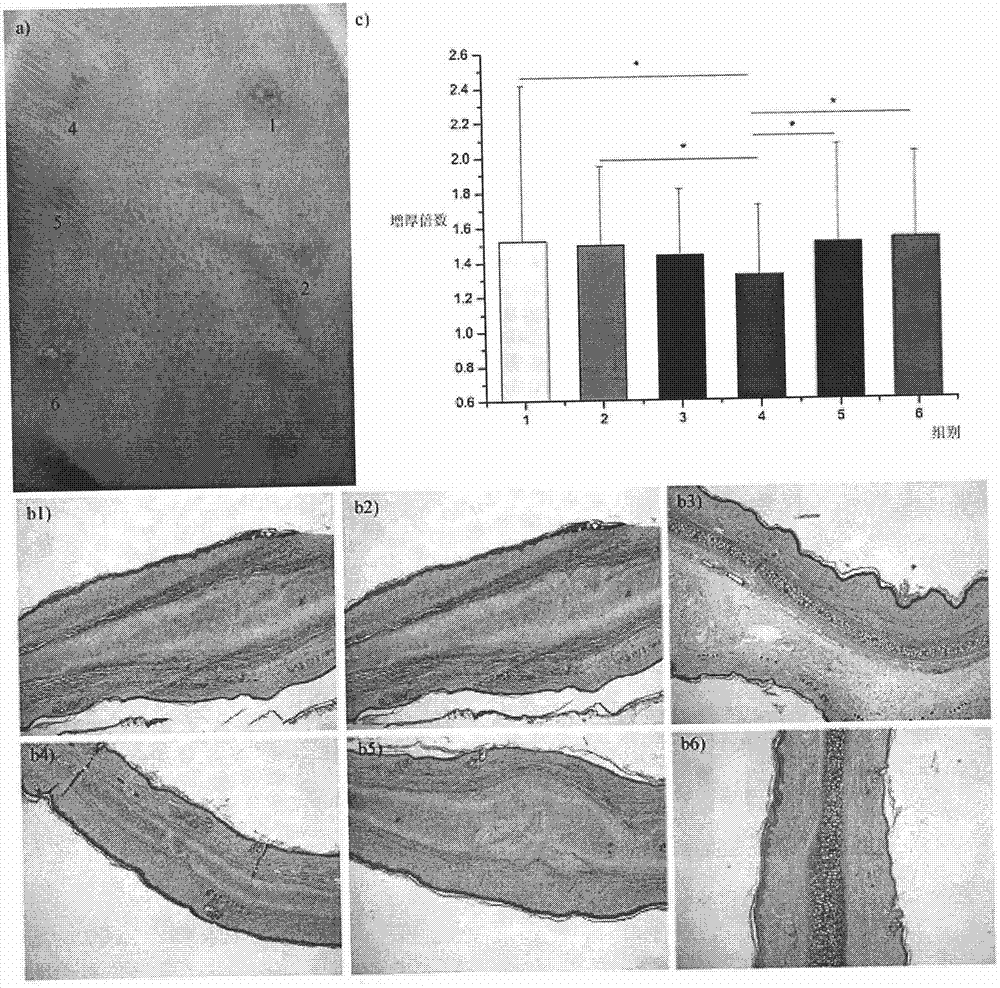
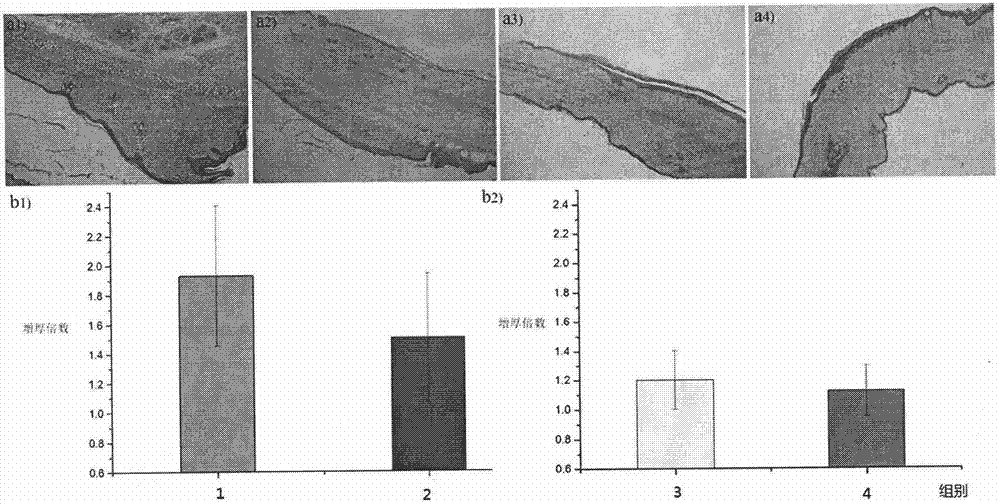
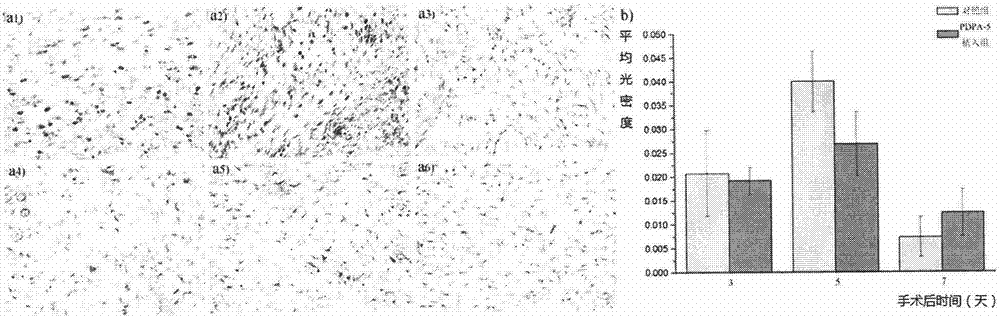
[0061] Experiment on the effect of preventing and treating scar hyperplasia in epidermal wounds
[0062] The precondition of the test is to use natural uninfected New Zealand rabbits or SD rats to carry out relevant experimental tests, divide the animals into random groups, 5 animals in each group, and test the experimental groups continuously.
[0063] 1) Circular incision on the inner skin of the rabbit ear 1
[0064] Use a circular drill to make a circular incision on the inner skin of the New Zealand rabbit's auricle to the cartilage layer, remove the cut circular skin, apply Example 1 prepared according to the method in Example 2 to the wound once a day within one week after the operation Polymer PDPA-5 powder / Bactobang ointment (stir the PDPA powder evenly into the Bactobang ointment, the following groups are made in the same way), and the control groups are: Bactobang ointment, L-phenylalanine / Bactobang ointment, D-phenylalanine / Bactobang ointment, no treatment bla...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com