Method for highly selectively synthesizing 2,3-dichlorobenzaldehyde
A technology of dichlorobenzaldehyde and high selectivity, which is applied in chemical instruments and methods, preparation of carbonyl compounds by hydrolysis, preparation of halogenated hydrocarbons, etc. It can solve problems such as difficulty in meeting product quality requirements, high environmental protection costs of processes, and difficulties in production control. , to achieve the effect of improving the utilization rate of atoms, making the process sustainable and reducing the generation of organic by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
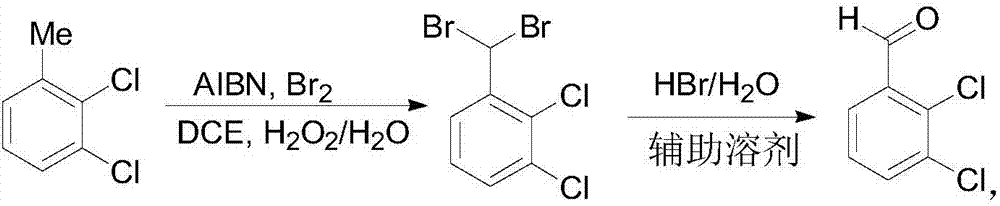
[0022] Add 97.8g of 2,3-dichlorotoluene, 360g of 1,2-dichloroethane and 3.9g of azobisisobutyronitrile into a 1000mL reactor, start stirring and heating, when the material temperature rises to 70°C, it starts to drop Add 110g of bromine, and simultaneously add 108g of 27.5% hydrogen peroxide dropwise, and the reaction temperature is stabilized at 80°C. After the completion of the reaction as detected by GC, the reaction solution was left to cool and separated into layers, the water phase was removed, and the organic phase was concentrated to obtain 180 g of 2,3-dichlorobenzylidene dibromide.
[0023] Put the product obtained by bromination into a 2000mL reaction kettle, add 360g of 9% hydrogen bromide aqueous solution, add 540g of auxiliary solvent N,N-dimethylacetamide, and heat up to 130-140°C for 8 hours for hydrolysis. The crude product of 2,3-dichlorobenzaldehyde was obtained by steam stripping, and 81.25 g of refined 2,3-dichlorobenzaldehyde was obtained after recrystall...
Embodiment 2
[0025] Add 97.8g of 2,3-dichlorotoluene, 360g of 1,2-dichloroethane and 4.1g of azobisisobutyronitrile into a 1000mL reactor, start stirring and heating, when the material temperature rises to 78°C, it starts to drop Add 111g of bromine, and simultaneously add 110g of 27.5% hydrogen peroxide dropwise, and the reaction temperature is stabilized at 85°C. After the reaction was detected by GC, the reaction was separated by standing and cooling, the water phase was removed, and the organic phase was concentrated to obtain 182 g of 2,3-dichlorobenzylidene dibromide.
[0026] Put the product obtained by bromination into a 2000mL reaction kettle, add 380g of 8% hydrogen bromide aqueous solution, add 550g of auxiliary solvent N,N-dimethylacetamide, heat up to 130-140°C for 8h for hydrolysis. The crude product of 2,3-dichlorobenzaldehyde was obtained by steam stripping, and 84 g of fine 2,3-dichlorobenzaldehyde was obtained after recrystallization, with a yield of 80.6% and a GC purity...
Embodiment 3
[0028] Add 97.8g of 2,3-dichlorotoluene, 350g of 1,2-dichloroethane and 4.8g of azobisisobutyronitrile into a 1000mL reactor, start stirring and heating, when the material temperature rises to 76°C, it starts to drop Add 113.2g of bromine, and simultaneously add 115g of 27.5% hydrogen peroxide dropwise, and the reaction temperature is stabilized at 85°C. After the completion of the reaction was detected by GC, the reaction was separated by standing and cooling, the water phase was removed, and the organic phase was concentrated to obtain 181.7 g of 2,3-dichlorobenzylidene dibromide.
[0029] Put the product obtained by bromination into a 2000mL reaction kettle, add 375g of 10% hydrogen bromide aqueous solution, add 575g of auxiliary solvent N,N-dimethylacetamide, heat up to 130-140°C for 8h for hydrolysis. The crude product of 2,3-dichlorobenzaldehyde was obtained by steam stripping, and 80.7 g of fine 2,3-dichlorobenzaldehyde was obtained after recrystallization, with a yield...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com