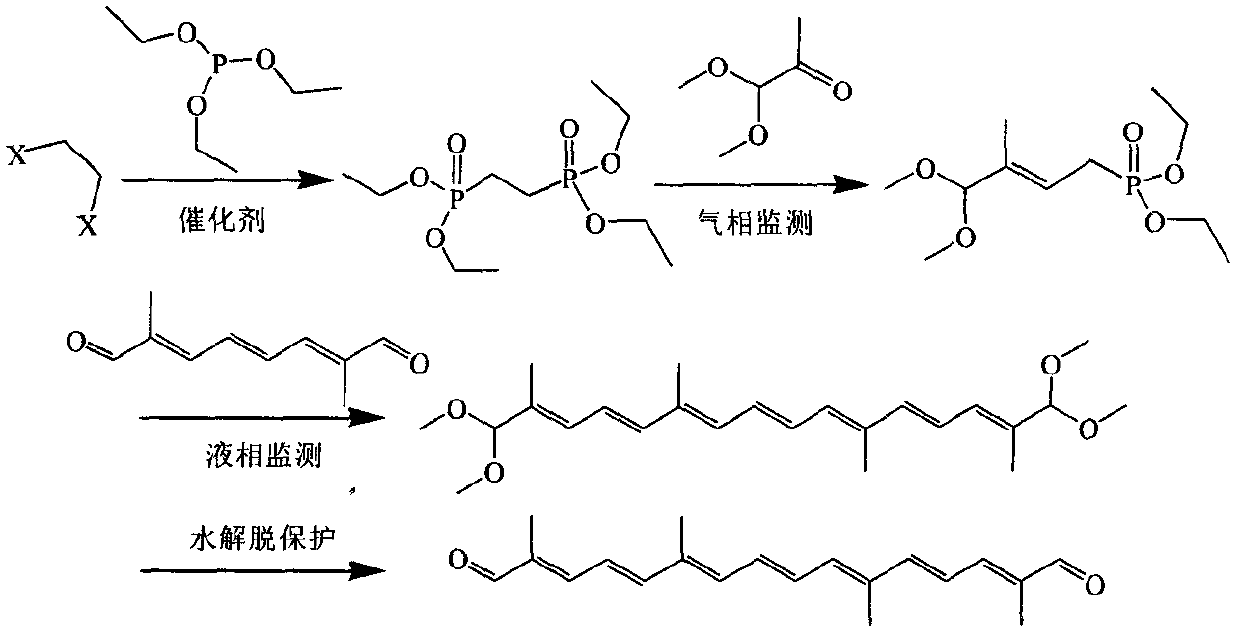
A kind of preparation method of 2,6,11,15-tetramethyl-2,4,6,8,10,12,14-hexadecadendial
A technology of carbheptaene dialdehyde and carbheptaene dialdehyde tetramethylene acetal is applied in the field of synthesis of carotenoid intermediates in organic chemistry, and can solve the problems of unobtainable raw materials, complicated operation, low yield and the like, and achieves yield. Good efficiency, simple operation, avoid side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] A. Preparation of tetraethyl ethylene diphosphate
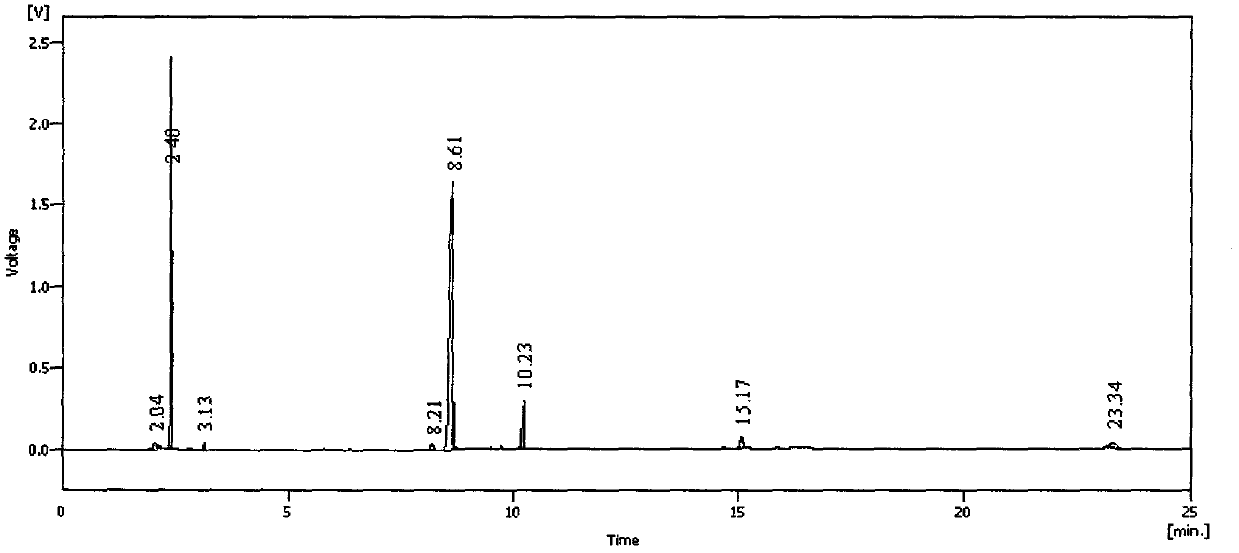
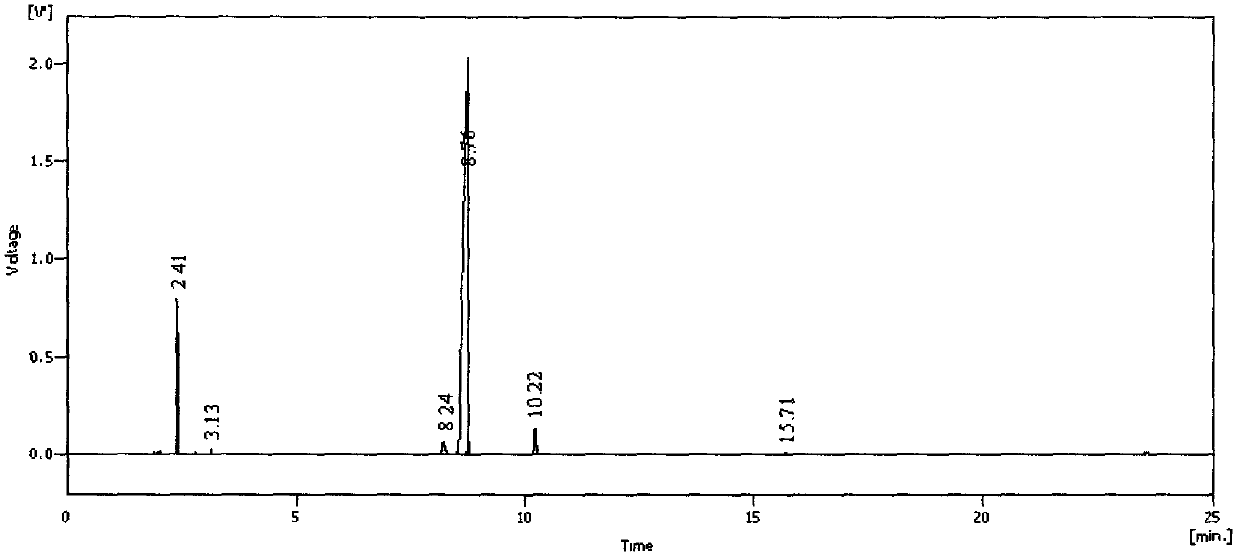
[0050] Add 18.8 g (0.1 mol) dibromoethane, 49.8 g (0.3 mol) triethyl phosphite, and 0.5 g tetrabutylammonium iodide to a dry 500 ml three-necked flask, and heat at 150-160° C. for reflux reaction 4 -5 h, the gas phase followed the reaction. After cooling down to 100°C, the unreacted raw material was distilled off by water pump under reduced pressure; the residue was crude product, and 26.3 g of product (about 120-125°C / 1mmHg) was obtained by vacuum distillation with oil pump. Gas phase analysis showed that the product content was 97%. Yield 87%.
[0051] B. "One-pot" preparation of 2,6,11,15-tetramethyl-2,4,6,8, 10,12,14-hexadecadedial
[0052] Under the protection of nitrogen, 150 ml of toluene and 16.8 g (0.15 mol) of potassium tert-butoxide solid were successively added to a dry 500 ml three-necked flask, and stirred evenly, and 36.3 g (0.12 mol) of tetraethyl ethylene diphosphate was added dropwise. , 11.8 g (0....
Embodiment 2
[0054] A. Preparation of tetraethyl ethylene diphosphate
[0055] Add 18.8 g (0.1 mol) dibromoethane, 49.8 g (0.3 mol) triethyl phosphite and 0.5 g nickel iodide to a dry 500 ml three-necked flask, and heat at 150-160°C for 4-5 h at reflux , followed by gas phase reaction. After cooling down to 100°C, the unreacted raw material was distilled out under reduced pressure by the water pump; the residue was a crude product, and 23.0 g of the product (about 120-125°C / 1mmHg) was obtained by vacuum distillation with an oil pump. The gas phase analysis showed that the product content was 96%, Yield 76%.
[0056] B. "One-pot" preparation of 2,6,11,15-tetramethyl-2,4,6,8, 10,12,14-hexadecadedial
[0057] Under the protection of nitrogen, 150 ml of toluene and 10.2 g (0.15 mol) of sodium ethoxide solid were successively added to a dry 500 ml three-necked flask, and the mixture was stirred evenly, and 36.3 g (0.12 mol) of tetraethyl ethylene diphosphate, 11.8 g (0.1 mol) of acetone alde...
Embodiment 3
[0059] A. Preparation of tetraethyl ethylene diphosphate
[0060] Add 18.8 g (0.1 mol) dibromoethane, 49.8 g (0.3 mol) triethyl phosphite and 0.5 g sodium iodide to a dry 500 ml three-necked flask, and heat at 150-160°C for 4-5 h at reflux , followed by gas phase reaction. After cooling down to 100°C, the unreacted raw material was distilled out by water pump under reduced pressure; the residue was crude product, and 21.7g of product (about 120-125°C / 1mmHg) was obtained by vacuum distillation with oil pump. Gas phase analysis showed that the product content was 96%. Yield 72%.
[0061] B. "One-pot" preparation of 2,6,11,15-tetramethyl-2,4,6,8, 10,12,14-hexadecadedial
[0062] Under the protection of nitrogen, 150 ml of toluene, 8.1 g (0.15 mol) of sodium methoxide solid were successively added to a dry 500 ml three-necked flask, and the mixture was stirred evenly, and 36.3 g (0.12 mol) of tetraethyl ethylene diphosphate, 11.8 g (0.1mol) of acetone aldehyde dimethyl acetal a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com