Preparation method for mushroom residue charcoal adsorbent and application thereof
A biochar and adsorbent technology, applied in chemical instruments and methods, adsorbed water/sewage treatment, water pollutants, etc., can solve problems such as non-point source pollution and resource waste, and achieve high adsorption efficiency, low cost, and raw material source. rich effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Preparation of fungus chaff biochar adsorbent:
[0026] (1) Put the fungus chaff into a constant temperature drying incubator at 80°C to dry to constant weight, crush it, and pass it through a 40-mesh sieve.
[0027] (2) Sieve the fungus chaff powder ( figure 1 ) were sterilized by high-pressure steam at 121°C and stored in a dry environment for later use.
[0028] (3) Put the fungus chaff powder in a carbonization furnace, use nitrogen as a protective gas, and carry out anaerobic carbonization at 600°C for 4 hours, take it out after cooling to room temperature, and pass through a 10-mesh sieve to obtain the fungus chaff biochar powder.
[0029] (4) Add 100 g of fungus chaff biochar powder into a conical flask containing 1L of deionized water, shake for 3 h in a shaker at a speed of 150 r / min, remove the aqueous solution after cleaning, and add deionized water again Wash and repeat 3 times to remove soluble minerals, and dry at 80°C to constant weight to obtain fungal...
Embodiment 2
[0031] Static adsorption of divalent copper ions by bacterial chaff biochar adsorbent:
[0032] Determination of the adsorption of fungal chaff biochar under different pH (2~5), initial concentration (25~150 mg / L), adsorbent dosage (0.5~4 g / L), adsorption time (30~300 min) Effect. After the adsorption is over, the atomic absorption spectrophotometer measures the copper ion content in the filtered aqueous solution. Calculate the adsorption rate R and adsorption capacity Q . Adsorption rate (%) and adsorption capacity Q (mg / g) Calculated according to the following formula:
[0033]
[0034] Where: C j Indicates the initial concentration of metal ions, mg / L;
[0035] C e Indicates the equilibrium concentration of metal ions, mg / L;
[0036] m represents the mass of the adsorbent, g;
[0037] V represents the volume of the metal ion solution used for adsorption, L.
[0038] Under the conditions of pH=5, the initial concentration of divalent copper ions was 50 mg / L, th...
Embodiment 3
[0040] Static adsorption of divalent lead ions by bacterial chaff biochar adsorbent:
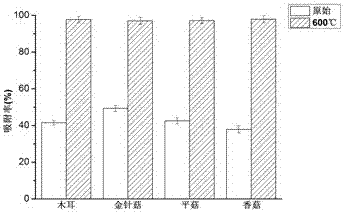
[0041] Under the conditions of pH=5, the initial concentration of divalent lead ions was 50 mg / L, the dosage of adsorbent was 2 g / L, the adsorption time was 120 min, the temperature was 25 °C, and the rotation speed was 180 r / min, the black The adsorption rates of fungus, Flammulina velutipes, Pleurotus ostreatus and Lentinus edodes biochar were 97.8%, 96.6%, 97.1% and 98.4%, and the adsorption amounts were 24.45 mg / g, 24.15 mg / g, 24.28 mg / g and 24.59 mg / g, respectively. g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com