Synthesis and uses of CETP inhibitor
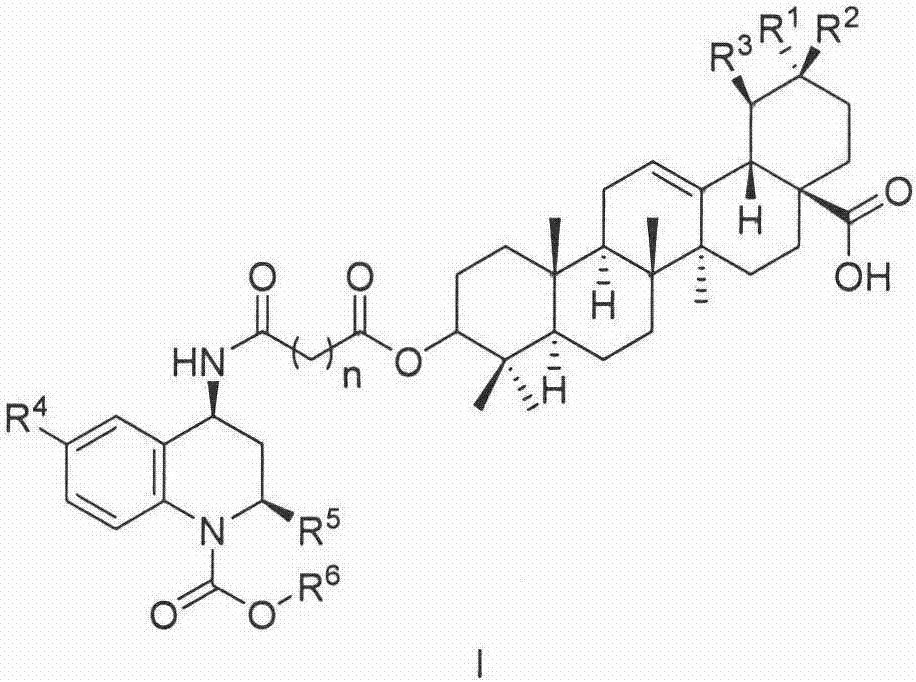
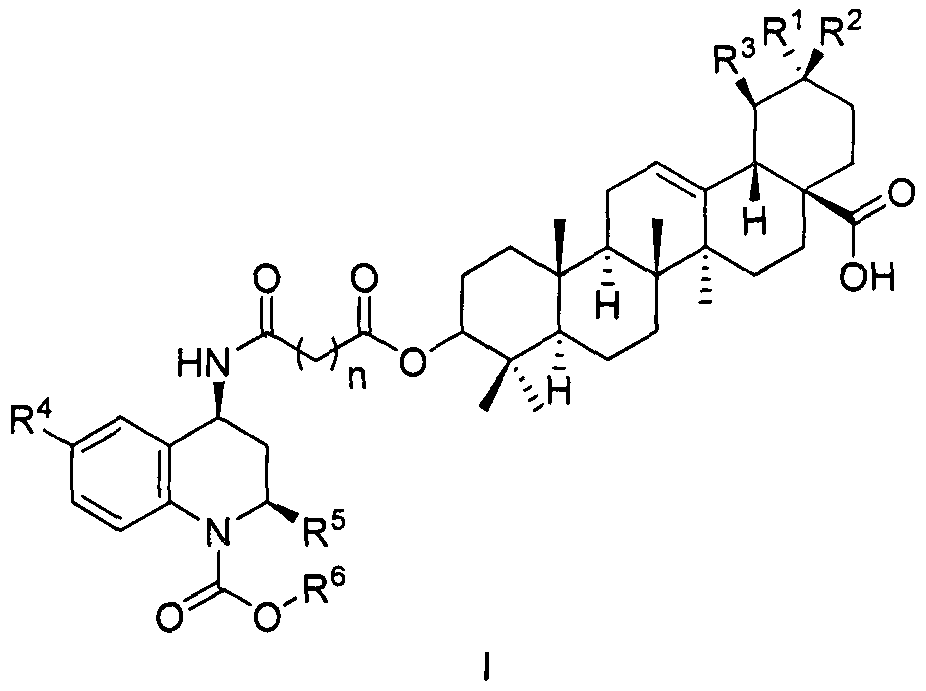
A compound and composition technology, applied in the fields of pentacyclic triterpenoid derivatives as CETP inhibitors, novel pentacyclic triterpenoid derivatives and their preparation, prevention and treatment of atherosclerosis and hyperlipidemia, capable of Solve the problems of not being able to effectively reduce cardiovascular and cerebrovascular adverse events, unknown cardiovascular diseases, etc., and achieve the effect of high application development value, cheap raw materials, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
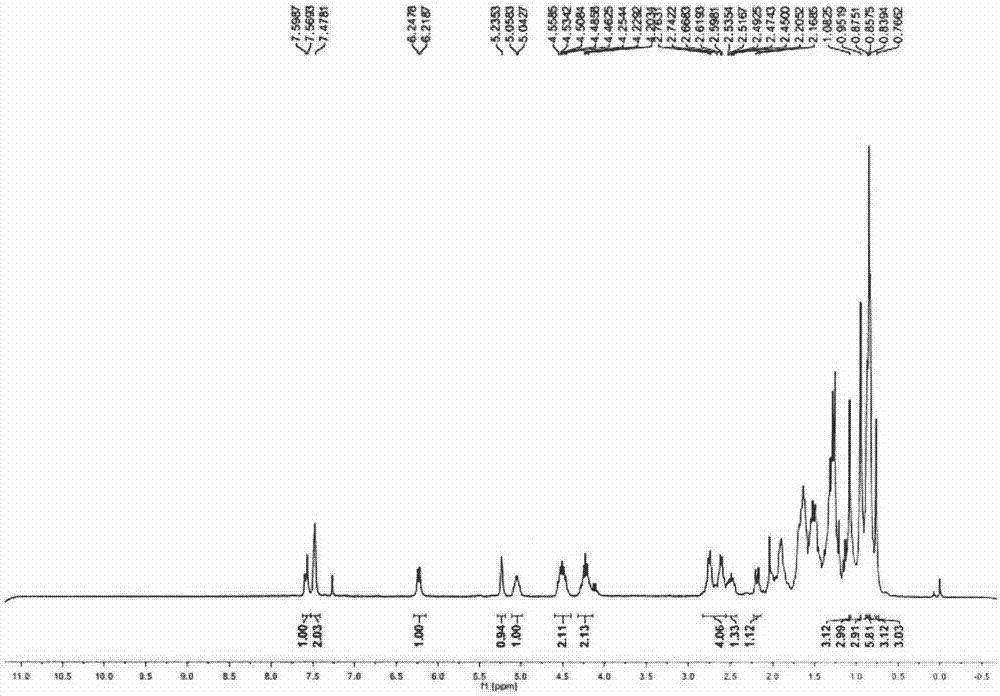
Embodiment 1
[0043] 3β-(3-(((2R,4S)-1-(ethoxycarbonyl)-2-ethyl-6-(trifluoromethyl)-1,2,3,4-tetrahydroquinoline-4 -yl)amino)-3-oxopropionyl)oxy-oleanane-12-en-28-acid (I-1)
[0044]
[0045] step one
[0046] At room temperature, benzyl oleanolic acid II-1 (200 mg) was dissolved in anhydrous toluene (3 mL), cyclo()isopropyl malonate V-1 (78 mg) was added, the temperature was raised to 120°C, and refluxed. 8 hours; the reaction solution was lowered to room temperature, poured into water (20 mL), the aqueous phase was extracted with ethyl acetate (10 mL x 3), the organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, and the organic phase was evaporated to dryness to obtain a crude product , by silica gel column chromatography (petroleum ether:ethyl acetate=6:1) to obtain 106 mg of benzyl oleanolic acid benzyl malonate monoester (III-1) as a light yellow viscous liquid with a yield of 46%.
[0047] Step 2
[0048] Compound III-1 (100 mg) was dissolved in dry...
Embodiment 2
[0052] 3β-(4-(((2R,4S)-1-(ethoxycarbonyl)-2-ethyl-6-(trifluoromethyl)-1,2,3,4-tetrahydroquinoline-4 -yl)amino)-4-oxobutyryl)oxy-oleanane-12-en-28-acid (I-2)
[0053]
[0054] step one
[0055] At room temperature, benzyl oleanolic acid II-1 (200 mg) was dissolved in anhydrous pyridine (3 mL), succinic anhydride V-2 (180 mg) and 4-dimethylaminopyridine (45 mg) were added successively, and the temperature was raised to 130 ° C, refluxed for 7 hours; the reaction solution was lowered to room temperature, water (20 mL) and dichloromethane (10 mL) were added respectively, first washed with 1.0 M hydrochloric acid (10 mL x 2), and then the organic phase was washed with water and saturated brine successively, It was dried over anhydrous sodium sulfate, and the organic phase was evaporated to dryness to obtain a crude product, which was subjected to silica gel column chromatography (petroleum ether: ethyl acetate = 6: 1) to obtain 230 mg of benzyl succinic acid benzyl oleanate mon...
Embodiment 3
[0061] 3β-(5-(((2R,4S)-1-(ethoxycarbonyl)-2-ethyl-6-(trifluoromethyl)-1,2,3,4-tetrahydroquinoline-4 -yl)amino)-5-oxopentanoyl)oxy-oleanane-12-en-28-acid (I-3)
[0062]
[0063] step one
[0064] At room temperature, benzyl oleanolic acid II-1 (200 mg) was dissolved in anhydrous pyridine (3 mL), glutaric anhydride V-3 (208 mg) and 4-dimethylaminopyridine (45 mg) were added successively, and the temperature was raised to 130 ° C, refluxed for 15 hours; the reaction solution was lowered to room temperature, water (20 mL) and dichloromethane (10 mL) were added respectively, first washed with 1.0 M hydrochloric acid (10 mL x 2), and then the organic phase was washed with water and saturated brine successively, It was dried over anhydrous sodium sulfate, and the organic phase was evaporated to dryness to obtain a crude product, which was subjected to silica gel column chromatography (petroleum ether:ethyl acetate=10:1) to obtain 222 mg of benzyl glutaric acid benzyl oleanolic ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com