Hyperbranched expanded type flame retardant and preparation method thereof
An intumescent flame retardant and flame retardant technology, which is applied in chemical instruments and methods, organic chemistry, compounds of Group 5/15 elements of the periodic table, etc., can solve the problem that the compatibility between flame retardants and substrates is not very good. , The flame retardant effect is not greatly improved, the synthesis steps of the flame retardant are cumbersome and other problems, to achieve the effects of high yield, reduced hygroscopicity, and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
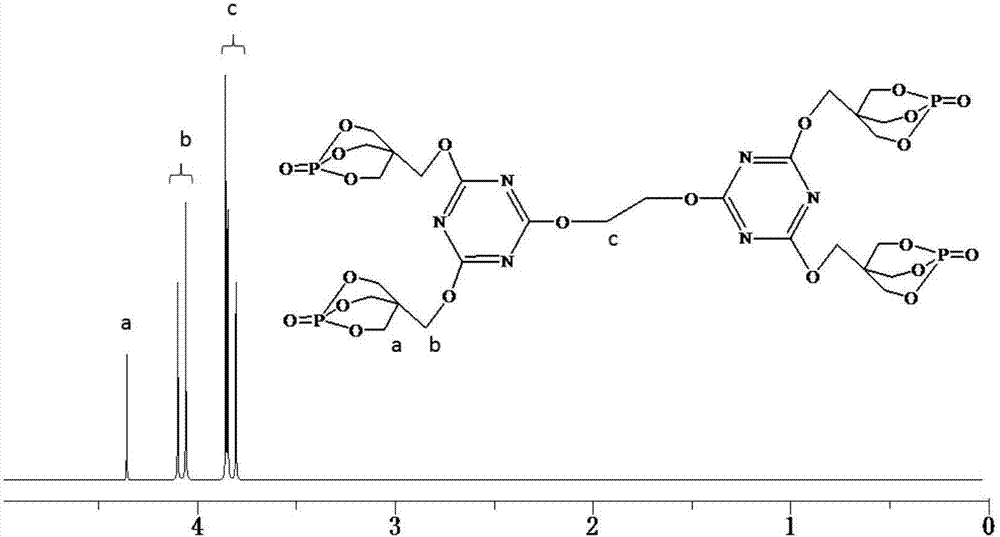
[0042] Add 22.14g (0.12mol) of cyanuric chloride and 250mL of dioxane into a 500mL three-necked round-bottomed flask equipped with a constant pressure dropping funnel, a serpentine condenser, and a thermometer, and stir the three with a magnetic force at 30°C. Polycyanide chloride is completely dissolved, and then 49.68g (0.276mol) of PEPA is slowly added to the flask. After half an hour of reaction, 24.24g (0.24mol) of triethylamine is slowly dropped into the round bottom flask with a constant pressure dropping funnel , The dropwise addition was completed within 1 hour, and then the reaction was continued for 1.5 hours to obtain a disubstituted product of cyanuric chloride. Continue to raise the temperature of the system to 70°C, add 3.72g (0.06mol) ethylene glycol to the round bottom flask, and then slowly drop 12.12g (0.12mol) triethylamine into the round bottom flask with a constant pressure dropping funnel In the flask, after reacting for 6 hours, cool the system to room ...
Embodiment 2
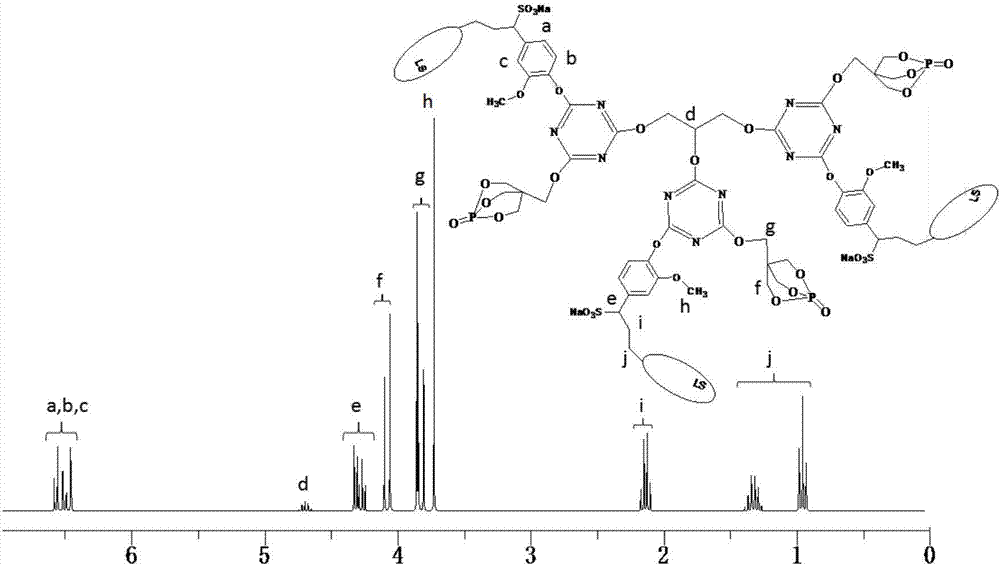
[0044]Add 22.14g (0.12mol) of cyanuric chloride and 250mL of dimethyl sulfoxide into a 500mL three-necked round-bottomed flask equipped with a constant pressure dropping funnel, a serpentine condenser, and a thermometer, and stir with magnetic force at 40°C. The cyanuric chloride is completely dissolved, and then 21.60g (0.12mol) of PEPA and 64.14g (0.12mol) of sodium lignosulfonate are slowly added to the flask, and after half an hour of reaction, 24.24g (0.24 mol) triethylamine is slowly added dropwise in the round-bottomed flask, and the dropwise addition is completed within 1.5 hours, and then the reaction is continued to obtain the disubstituted product of cyanuric chloride after 3 hours. Continue to raise the temperature of the system to 85°C, add 3.68g (0.04mol) glycerol to the round bottom flask, then slowly add 12.12g (0.12mol) triethylamine dropwise to the round bottom flask with a constant pressure dropping funnel In the flask, after reacting for 4 hours, the system...
Embodiment 3
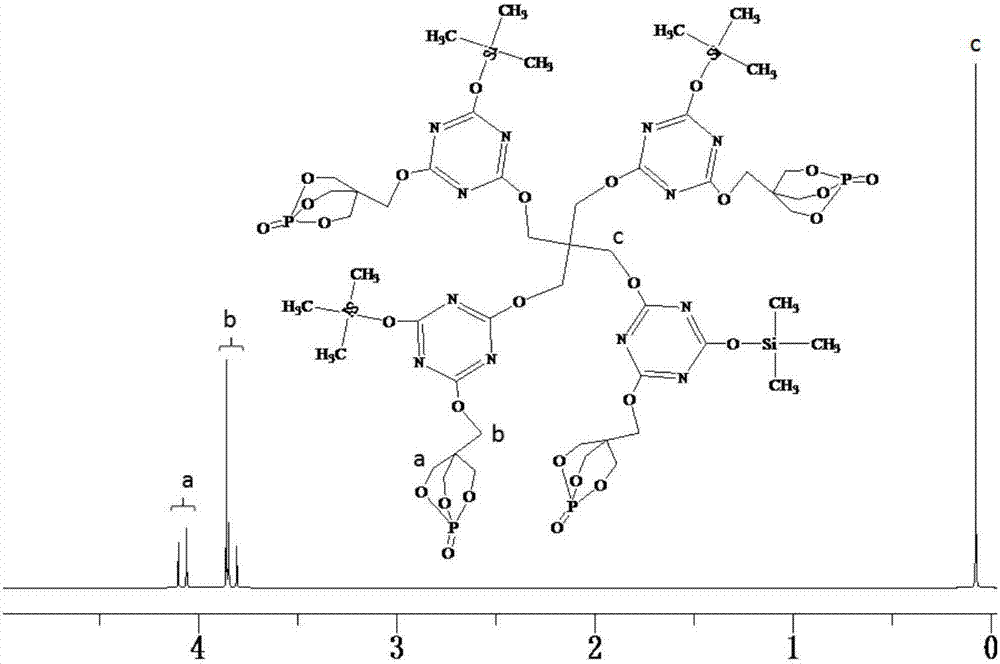
[0046] Add 22.14g (0.12mol) of cyanuric chloride and 250mL of dimethylformamide into a 500mL three-necked round-bottomed flask equipped with a constant pressure dropping funnel, a serpentine condenser, and a thermometer, and stir with magnetic force at 50°C. Melamine chloride is completely dissolved, and then 21.60g (0.12mol) of PEPA and 13.44g (0.12mol) of trimethylsilanol are slowly added to the flask, and after half an hour of reaction, 24.24g (0.24 mol) triethylamine is slowly added dropwise in the round-bottomed flask, and the dropwise addition is completed within 1.5 hours, and then the reaction is continued to obtain the disubstituted product of cyanuric chloride after 4 hours. Continue to raise the temperature of the system to 95°C, add 3.944g (0.029mol) of pentaerythritol into the round bottom flask, then slowly drop 12.12g (0.12mol) of triethylamine into the round bottom flask with a constant pressure dropping funnel After reacting for 3 hours, the system was cooled ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com