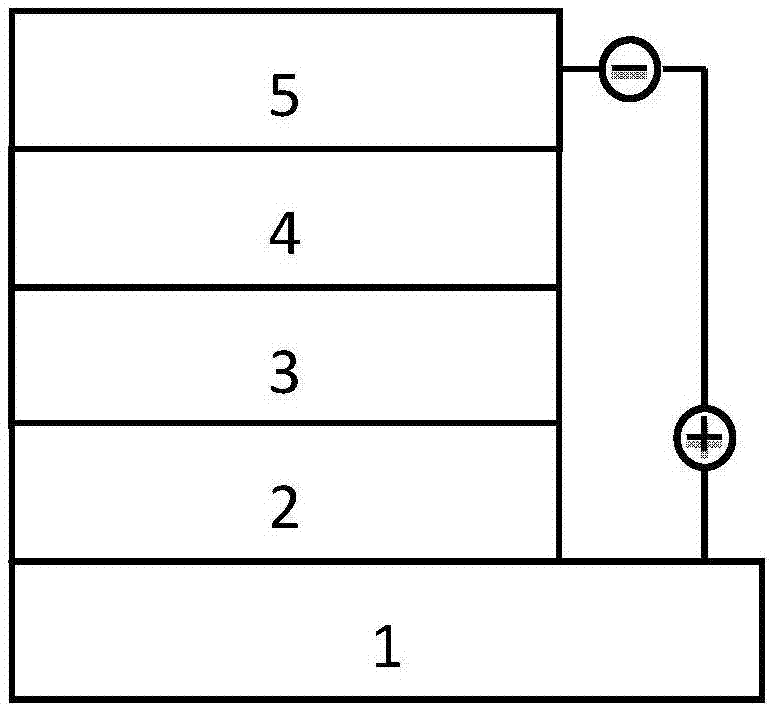
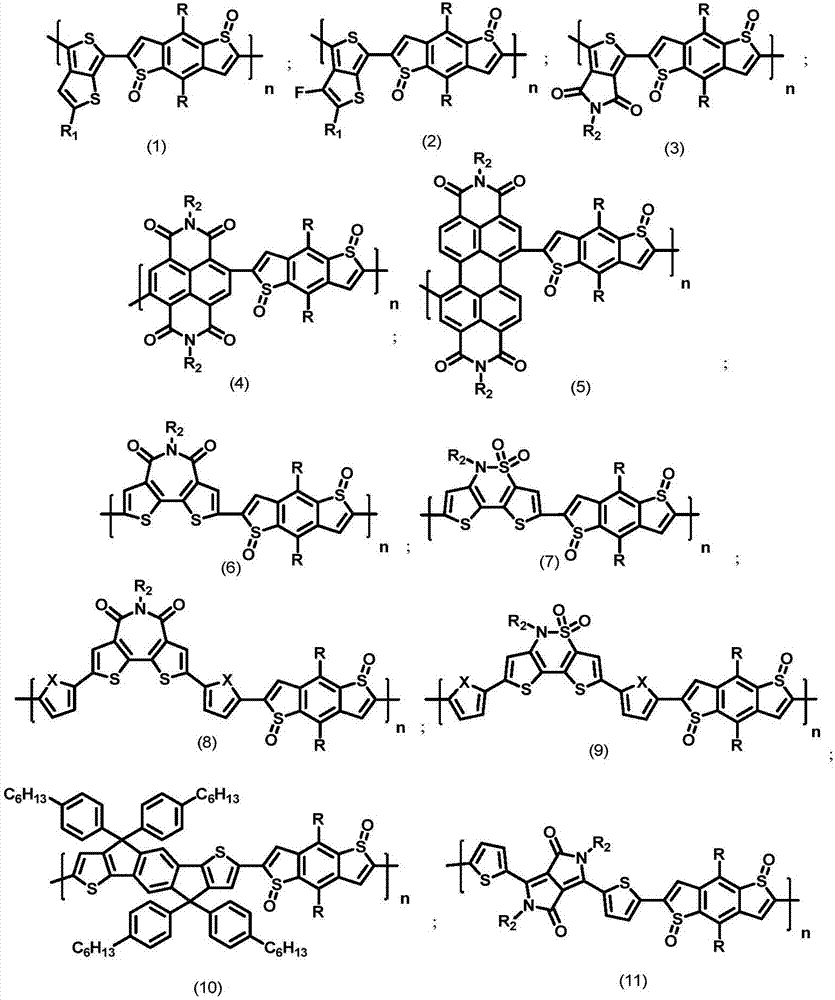
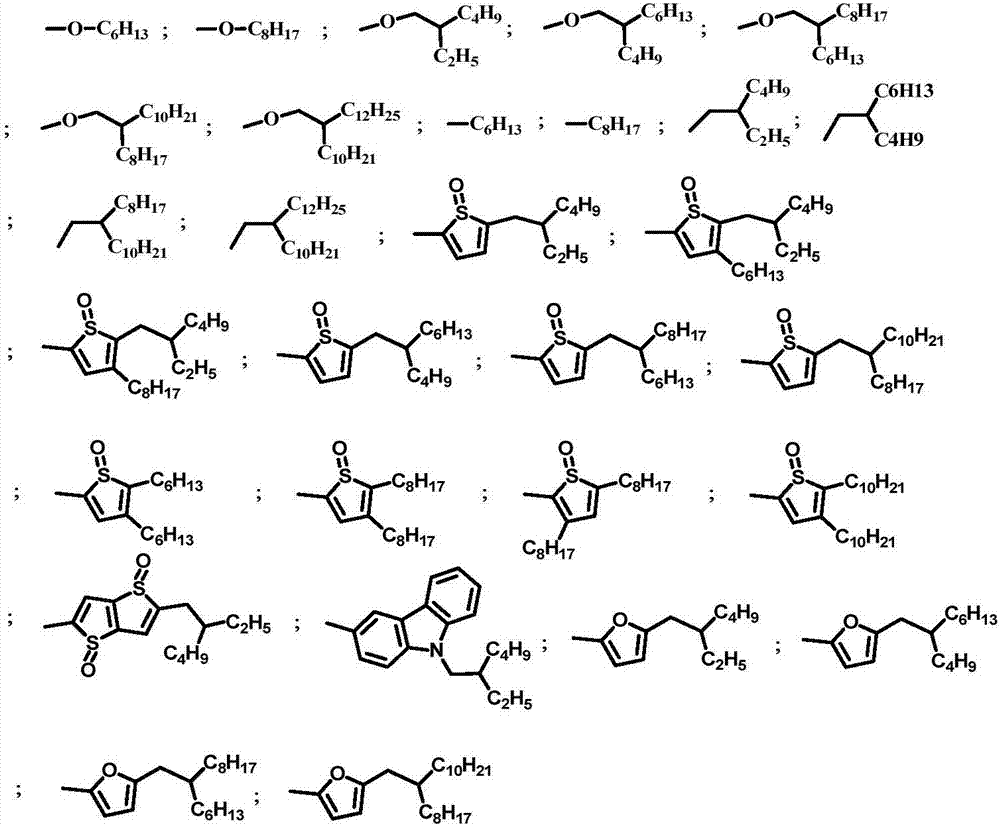
Benzo disulfoxide thienyl polymer, preparation method and application thereof
A technology of benzodisulfoxide and polymers, which is applied in the field of benzodisulfoxide phenyl polymers, can solve the problems of low carrier mobility, narrow absorption spectrum, and poor stability, and achieve broad absorption spectrum and enhanced Effect of Molar Absorption Coefficient and Coplanarity Improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] In the present embodiment, the polymer structural formula is as follows:
[0031]
[0032] The preparation of this polymer comprises the steps:
[0033] (1) Synthesis of benzodisulfoxide phenyl acceptor unit
[0034]
[0035] Add compound 1 (0.5g), bis-boronate (1.02g) and appropriate amount of potassium acetate into a three-necked flask, replace nitrogen three times, add catalyst [1,1'-bis(diphenylphosphino)ferrocene] di Palladium chloride (50 mg), and nitrogen was replaced three times, and finally 50 mL of dry 1,4-dioxane was added to react under reflux for 12 hours. After the reaction was completed, the solvent was removed with a rotary evaporator, then extracted with chloroform and water, and dried with anhydrous sodium sulfate after extraction. After being spin-dried, it was passed through a silica gel column to obtain a light yellow product 2 with a mass of 0.57 g and a yield of 97%.
[0036] 1H-NMR (400MHz, CDCl3) of the product: δ6.9(s, 2H), 4.03(t, 2H)...
Embodiment 2
[0046] In this example, the polymer is the same as that of Example 1.
[0047] The polymer was prepared using stille coupling polymerization:
[0048]
[0049] Add compound 1 (100 mg), compound 5 (134 mg), tetrakistriphenylphosphine palladium (4 mg) and tris(dibenzylideneacetone) dipalladium (4 mg) into the polymerization tube, vacuumize and fill with nitrogen three times, and add 5 ml of toluene , reacted at 85 degrees Celsius for 48 hours, and then added an appropriate amount of phenyl borate and bromobenzene for capping. After the reaction was completed, the precipitate was poured into methanol, filtered, and dried. Finally, the product was subjected to Soxhlet extraction, respectively using methanol, acetone and n-hexane as solvents, and finally using toluene to obtain 48 mg of polymer product 6 with a yield of 35%.
[0050] 1H-NMR (400MHz, CDCl3) of the product: δ8.05(d,1H),7.81(d,1H),7.68(d,1H),7.3(s,1H),7.18(d,1H ),6.7(s,1H),4.03-3.77(d,4H),3.04-2.79(d,4H),2.10-1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com