Method for preparing isradipine impurities I
A technology for isradipine and impurities, which is applied in the field of preparation of isradipine impurity I, can solve the problems of difficulty in separation and affect the quality of the final product isradipine, and achieves the effects of easy availability of raw materials, high product purity and simple preparation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
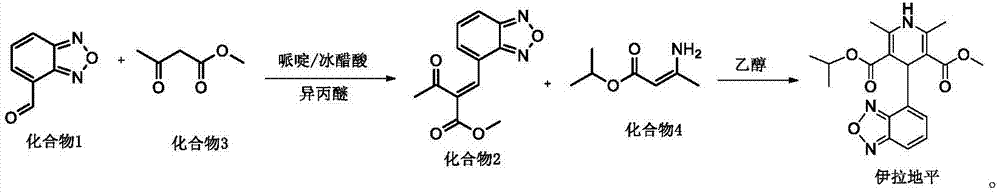
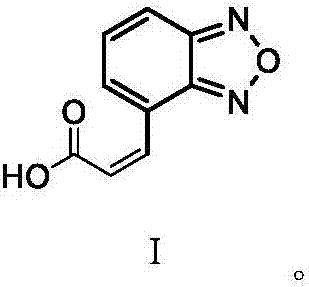
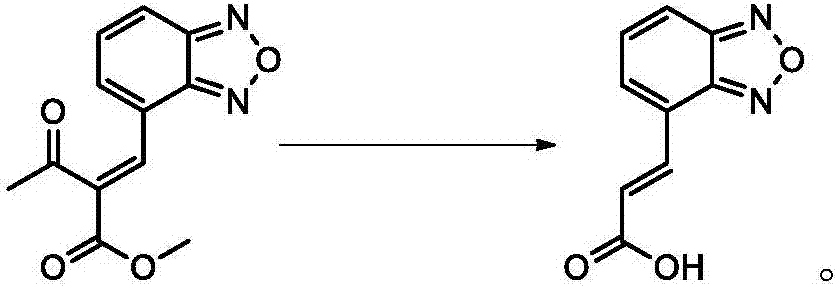
[0030] The invention provides a preparation method of isradipine impurity I, which is characterized in that: 2-acetyl-3-benzofurazan-4-yl-methyl acrylate is used as a raw material, and desorption occurs under alkaline organic conditions. Acetyl reaction, the isradipine impurity I is obtained after separation, the reaction formula is as follows:
[0031]
[0032] Further, the preparation method of the isradipine impurity I specifically includes the following steps:
[0033] S1. Add 5-10g of 2-acetyl-3-benzofurazan-4-yl-methyl acrylate into 120-250ml of organic solvent and stir. After fully dissolving, add 10-30ml of alkali solution to carry out temperature-raising reflux reaction;
[0034] S2, the reaction liquid obtained in step S1 is concentrated under reduced pressure, and the low boiling point components are removed;
[0035] S3. Adjust the concentrated solution of step S2 to PH=3 with concentrated hydrochloric acid, add 30-80 ml of ethyl acetate for extraction, separat...
Embodiment 1
[0048] Add 5g of compound 2 and 125mL of anhydrous methanol to the reaction bottle, stir and dissolve, add 10mL of 5% sodium hydroxide solution after fully dissolved, heat up and reflux for 10h, concentrate under reduced pressure to remove low boiling point components; adjust the concentrated solution to PH=3 with concentrated hydrochloric acid , add 30mL ethyl acetate for extraction, separate the layers to obtain the organic layer, add 5g of anhydrous sodium sulfate to dry, filter, and concentrate the filtrate; : n-hexane=2:1); Concentrate the purified solution, add 5 mL of ethyl acetate to heat up to dissolve, cool down to 5°C to crystallize, filter, and dry at 60°C to obtain impurity Ⅰ. Impurity I has a purity of 99.2%, a weight of 2.28 g, and a yield of 59.1%.
[0049] The mass spectrum data and NMR data detection of the product obtained in this embodiment are as follows: ESI-MS: 189.16 [M-H] -;1H-NMR: (δ-DMSO), δ7.076~7.102(1H,d), δ7.637~7.664(1H,dd), δ7.793~7.820(1H,d),...
Embodiment 2
[0051] Add 5g of compound 2, 150mLN, and N-dimethylformamide to the reaction flask, stir and dissolve, add 15mL of 5% potassium hydroxide solution after fully dissolving, heat up and reflux for 12h, concentrate under reduced pressure to remove low boiling point components; use concentrated hydrochloric acid to adjust the concentration solution to PH=3, add 30mL ethyl acetate for extraction, separate the layers to obtain an organic layer, add 10g of anhydrous sodium sulfate to dry, filter, and concentrate the filtrate; the concentrate is separated and purified by column (the eluent is a combination of ethyl acetate and n-hexane product, ethyl acetate: n-hexane = 2:1); concentrate the purified solution, add 5 mL of tetrahydrofuran to dissolve it at elevated temperature, cool down to 0°C to crystallize, filter, and dry at 60°C to obtain impurity Ⅰ. Impurity I has a purity of 99%, a weight of 2.56 g, and a yield of 66.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com