Preparation method for water-soluble zinc sulfide quantum dots
A technology of zinc sulfide and quantum dots, which is applied in the direction of zinc sulfide, chemical instruments and methods, nanotechnology, etc., can solve the problems of difficult particle size control, cumbersome operation steps, and high equipment requirements, and achieve environmental friendliness, cheap and easy-to-get raw materials, and strong Effect of Fluorescent Properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
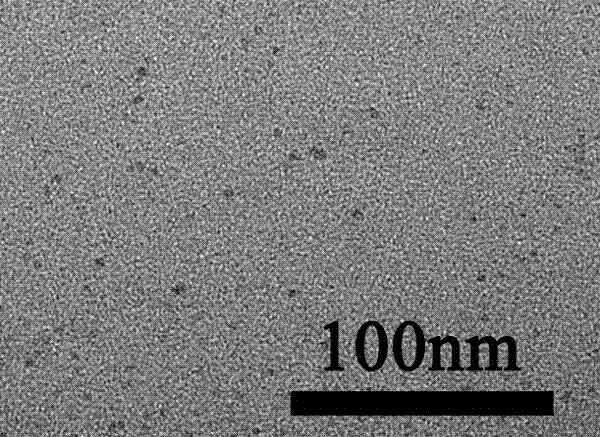
[0016] 1.4874g of zinc nitrate was dissolved in 200mL of bovine serum albumin solution with a mass concentration of 5g / L to obtain a chelating solution of zinc ions; 4.0g of thioacetamide was dissolved in 100mL of acetic acid solution to obtain a sulfur source solution; The chelating solution and the sulfur source solution were quickly sealed in two different containers in the same closed reaction system at a volume ratio of 1:2, and then the closed reaction system was reacted at 4°C for 72h. After the reaction, it was placed in a centrifuge at 1300r Centrifugation at a centrifugal rate of / min, the centrifuged product was alternately washed three times with absolute ethanol and high-purity water, and dried to obtain water-soluble zinc sulfide quantum dots.
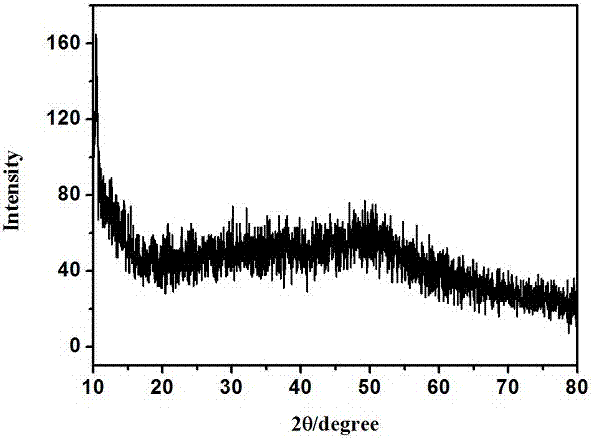
[0017] figure 1 It is the X-ray diffraction spectrum of the water-soluble zinc sulfide quantum dots that the present embodiment makes, as can be seen from the figure, there is no obvious peak, indicating that the product ...
Embodiment 2
[0019] 1.4874g zinc nitrate is dissolved in the bovine serum albumin solution that 200mL mass concentration is 0.1g / L to obtain the chelation solution of zinc ion; 4.0g thioacetamide is dissolved in 100mL sulfuric acid solution to obtain sulfur source solution; Zinc The chelating solution of ions and the sulfur source solution were quickly sealed in two different containers in the same closed reaction system at a volume ratio of 1:1, and then the closed reaction system was reacted at 8°C for 72 hours, and placed in a centrifuge after the reaction was completed. Centrifuge at a centrifugal rate of 1300r / min, and the centrifuged product is alternately washed three times with absolute ethanol and high-purity water, and dried to obtain water-soluble zinc sulfide quantum dots.
Embodiment 3
[0021] 1.4874g zinc nitrate is dissolved in the bovine serum albumin solution that 200mL mass concentration is 10g / L to obtain the chelation solution of zinc ion; 4.0g thioacetamide is dissolved in the 100mL nitric acid solution to obtain sulfur source solution; Zinc ion The chelating solution and the sulfur source solution were quickly sealed in two different containers in the same closed reaction system at a volume ratio of 1:4, and then the closed reaction system was reacted at 4°C for 72h. After the reaction, it was placed in a centrifuge at 1300r Centrifugation at a centrifugal rate of / min, the centrifuged product was alternately washed three times with absolute ethanol and high-purity water, and dried to obtain water-soluble zinc sulfide quantum dots.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com