A kind of bis-(2-aminoacetyl)-piperazine amide derivative lubricating oil additive and its preparation method
The technology of lubricating oil additive and aminoacetyl is applied in the field of bis-piperazine amide derivative lubricating oil additive and its preparation, and achieves the effects of good extreme pressure, anti-wear and friction reduction, simple preparation process and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] Preparation of Intermediate 1:
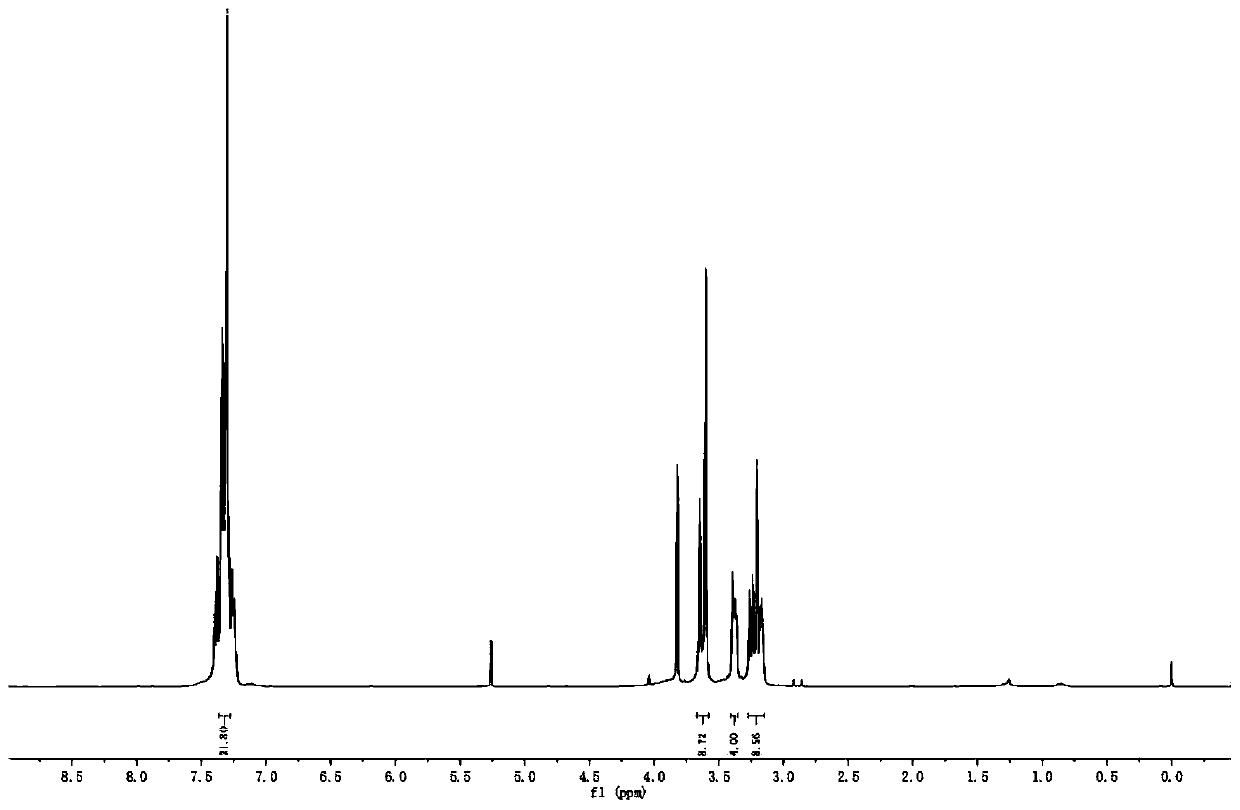
[0034] Add 8.6g of piperazine, 150mL of chloroform and 100mL of saturated potassium carbonate aqueous solution into a 500mL three-neck flask, add 24.8g of chloroacetyl chloride dropwise under ice cooling, and stir at room temperature for 3h. Stop the reaction, transfer the reaction solution to a separatory funnel, separate the organic phase, wash twice with 1M hydrochloric acid solution, wash three times with saturated brine, dry over anhydrous magnesium sulfate, filter, and remove the organic solvent to obtain Intermediate 1.
Embodiment 1
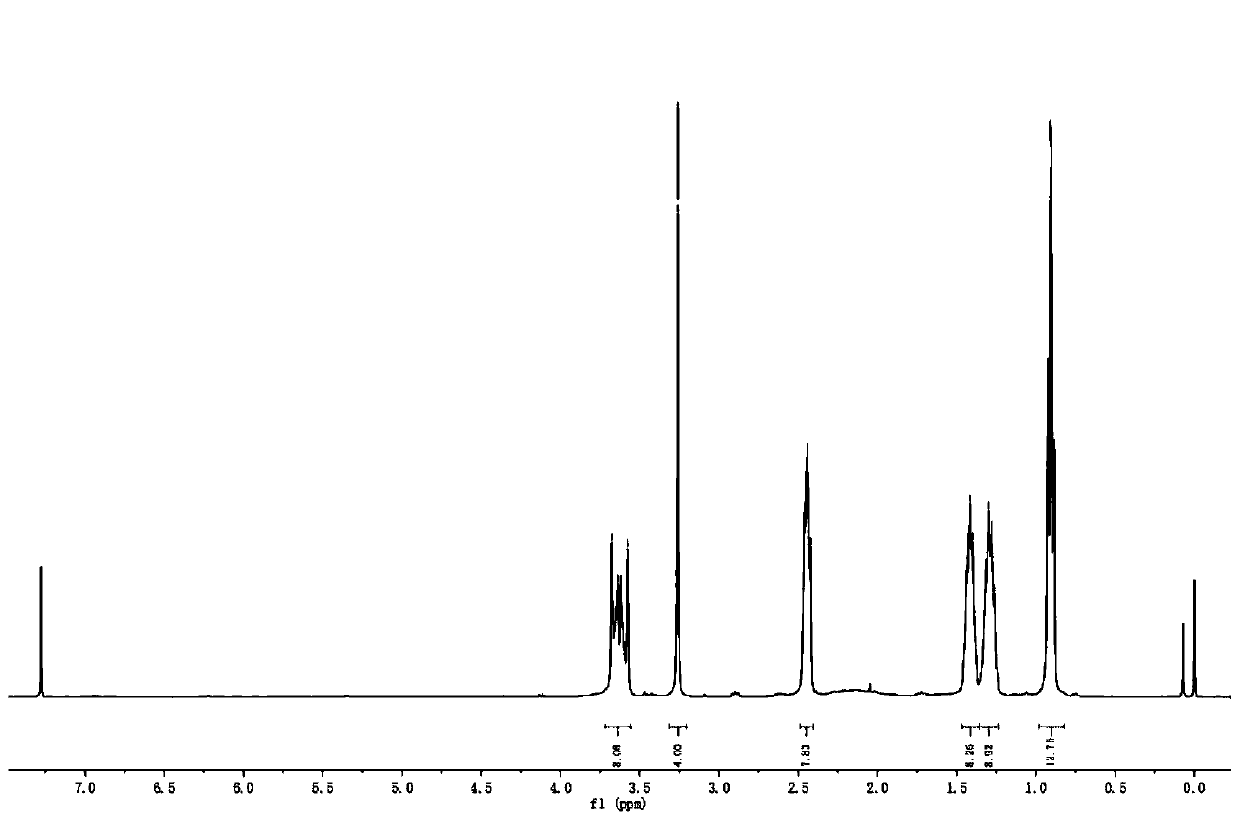
[0036] Add 15.5g of di-n-butylamine, 11.9g of intermediate 1, 12.6g of sodium bicarbonate and 100mL of acetonitrile into a 250mL three-necked flask, and react at 65°C for 8h. Stop the reaction, cool to room temperature, filter to remove insoluble matter, remove the solvent by rotary evaporation, dissolve with dichloromethane, wash with saturated brine, dry over anhydrous magnesium sulfate, filter, and remove the solvent by rotary evaporation of the filtrate to obtain 19.3 g of a light yellow liquid. The rate is 91.0%. 1 H NMR (400MHz, CDCl 3 ,TMS,ppm)δ:3.72–3.56(m,8H),3.26(s,4H),2.45(dd,J=12.4,5.7Hz,8H),1.47–1.36(m,8H),1.29(dd, J=14.5,7.2Hz,8H),0.93–0.88(m,12H).MALDI-TOF-MS,m / z:calcd for C 24 h 48 N 4 o 2 [M+1] + :425.378,found:425.355.
Embodiment 2
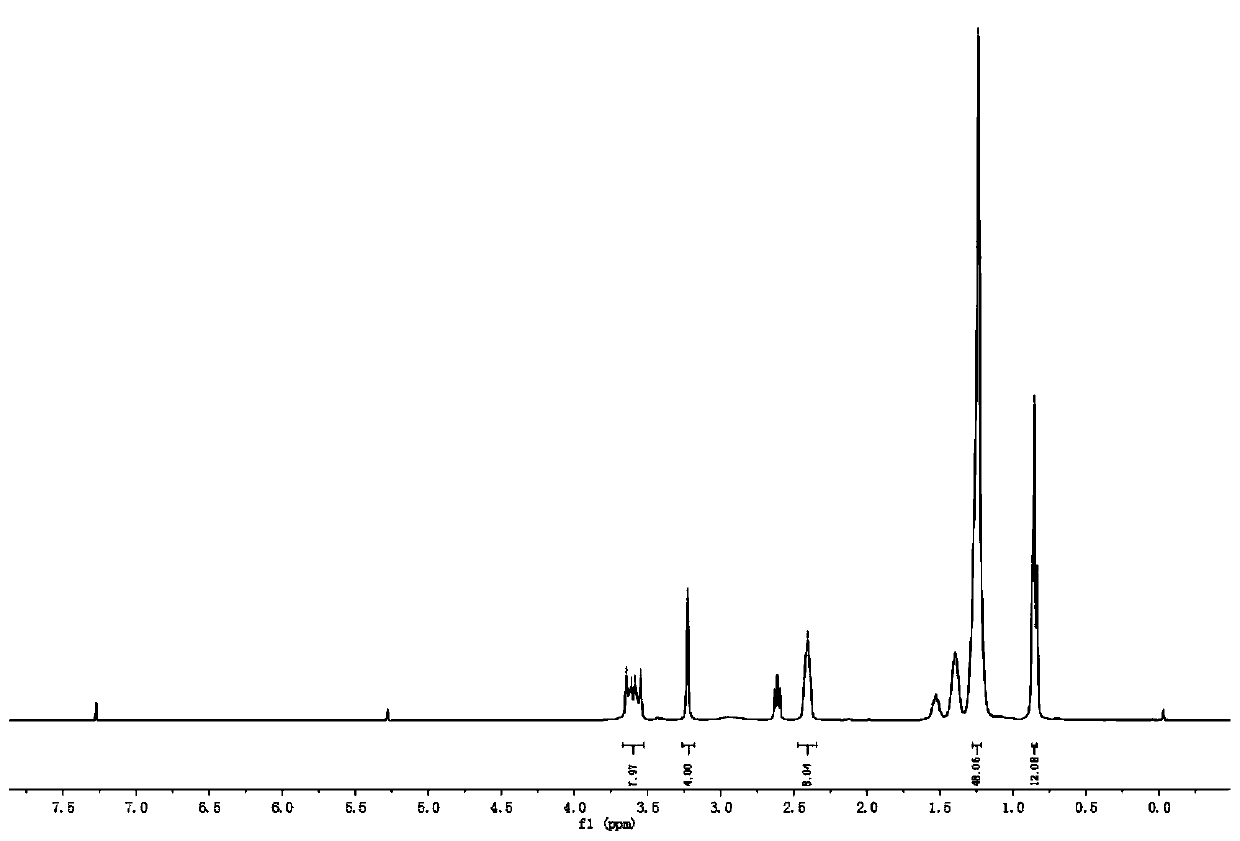
[0038] Add 27.0g of dioctylamine, 11.9g of intermediate 1, 12.6g of sodium bicarbonate and 100mL of acetonitrile into a 250mL three-necked flask, and react at 65°C for 8h. Stop the reaction, cool to room temperature, remove the insoluble matter by filtration, remove the solvent by rotary evaporation, dissolve with dichloromethane, wash with saturated brine, dry over anhydrous magnesium sulfate, filter, and remove the solvent by rotary evaporation of the filtrate to obtain 29.4 g of light yellow liquid. The rate is 90.7%. 1 H NMR (400MHz, CDCl 3 ,TMS,ppm)δ:3.60(dd,J=24.6,15.4Hz,8H),3.23(s,4H),2.40(s,8H),1.24(s,48H),0.85(s,12H).MALDI -TOF-MS, m / z: calcd for C 40 h 80 N 4 o 2 [M+1] + :649.628,found:649.735.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com