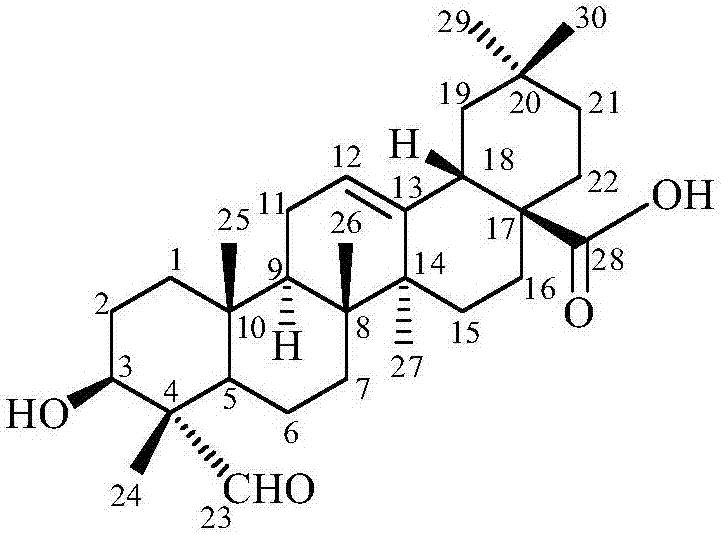
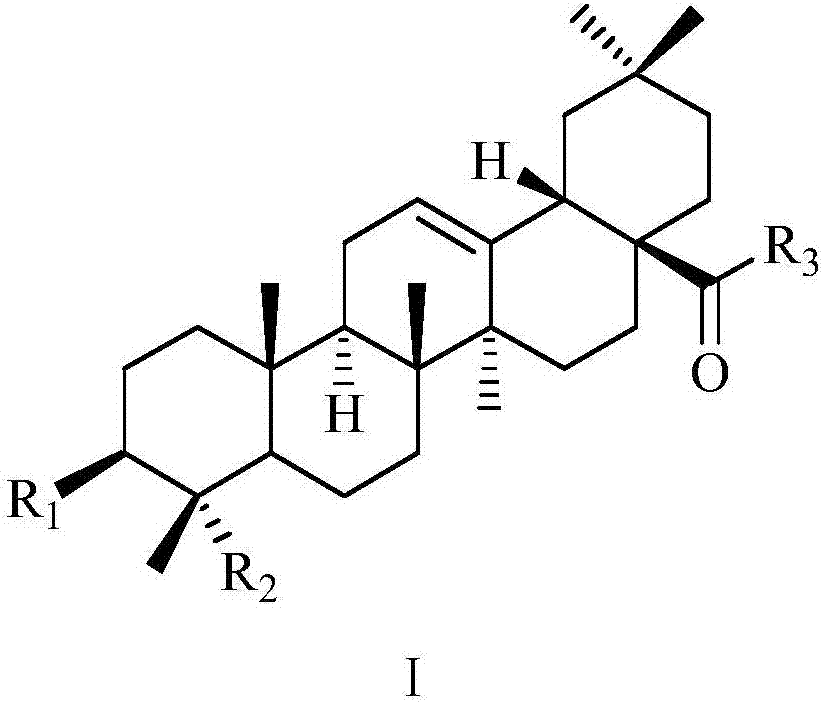
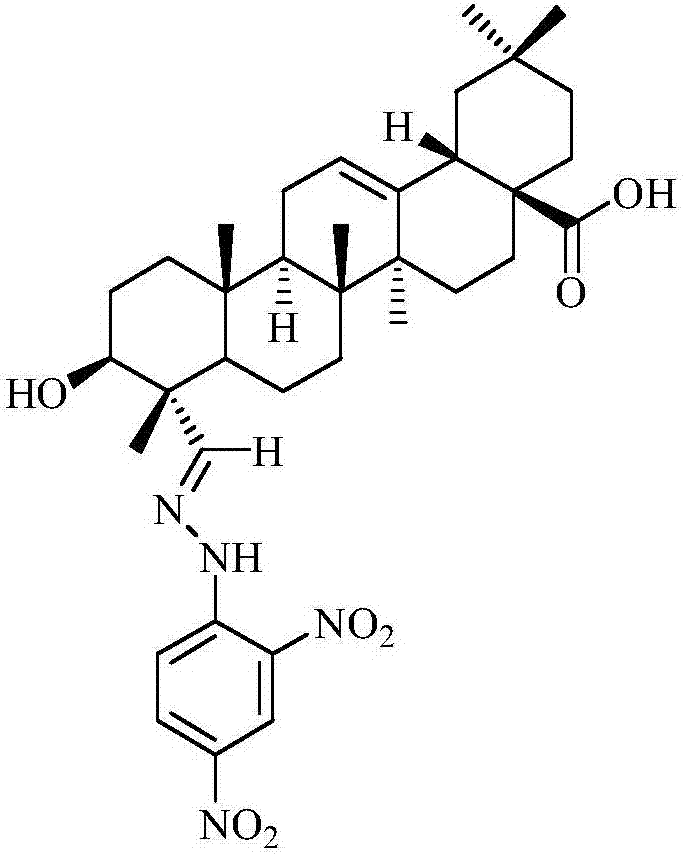
Gypsogenin derivatives
A technology of carnation soap and derivatives, applied in the field of medicine, can solve the problems of high molecular polarity, reduced bioavailability, low fat solubility and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Preparation of 3β-hydroxy-oleanane-12-ene-23-(2,4-dinitrophenylhydrazone)-28-carboxylic acid (compound 2):
[0065] The dianthin (100mg, 2.12×10 -4 mol) and 2,4-dinitrophenylhydrazine (63mg, 3.18×10 - 4 mol) was dissolved in 5 mL of glacial acetic acid and stirred at room temperature for 4 h. The end point of the reaction was controlled by TLC. After the reaction, 20 mL of distilled water was added, followed by ethyl acetate for extraction (3×10 mL), the extract was washed with water, the organic phases were combined, dried over anhydrous sodium sulfate, and dried under reduced pressure to obtain crude compound 2. The crude product was purified by silica gel column chromatography, and the eluent was chloroform / methanol=100 / 2 (V / V), to obtain 107.35 mg of a yellow powdery solid, with a yield of 77.9%; mp 206-207.1°C; IR(KBr)νmax: 3452 ,3302,3101,2941,2860,2853,1697,1618,1589,1519,1463,1425,1386,1332,1277,1216,1139,1075,958,920,832,712,646,580,526,454cm -1 ; LC / MS (ES...
Embodiment 2
[0067] Preparation of N-[3β-acetoxy-oleanane-12-en-23-aldehyde-28-yl]-piperidine (compound 4a):
[0068] Carnation (94mg, 2.0×10 -4 mol) and DMAP (2.44mg, 2.0×10 -5 mol) was dissolved in 5 mL of pyridine, and acetic anhydride was added with stirring at room temperature, and the end point of the reaction was controlled by TLC. After 24 hours, the reaction was completed, and the reaction liquid was transferred to a mixture of ice and water, a white solid precipitated out, extracted with ethyl acetate (3×10 mL), the extract was washed with water, the organic phases were combined, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain the crude product Compound 3, the crude product was purified by silica gel column chromatography, and the eluent was chloroform / acetone=100 / 1 (V / V) to obtain pure compound 3. Compound 3 (40mg, 7.8×10 -5 mol) was dissolved in 3 mL of dry dichloromethane, and oxalyl chloride (2.0×10 -1 mL, 36×10 -3 mol), after 12h, ...
Embodiment 3
[0070] Preparation of N-[3β-acetoxy-oleanane-12-en-23-aldehyde-28-yl]-morpholine (compound 4b):
[0071] Compound 3 (40mg, 7.8×10 -5 mol) was dissolved in 3mL of dry dichloromethane, and oxalyl chloride (2.0×10-1mL, 2.36×10 -3 mol), after 12h, terminate the reaction, evaporate the solvent to dryness under reduced pressure, add dichloromethane to dissolve, evaporate the solvent to dryness, repeat the operation three times, then add dichloromethane to dissolve, stir at room temperature and add triethylamine successively, adjust the pH to neutral After inactivation, add morpholine (3.52×10 -2 mL, 4.04×10 -4 mol), continue to stir for 8h, and TLC controls the reaction end point. After the reaction, adjust the pH to 3-4 with 2mol / L hydrochloric acid aqueous solution, add saturated aqueous sodium chloride solution, wash the organic phase with water, combine the organic phase, dry over anhydrous sodium sulfate, and concentrate under reduced pressure to obtain the crude compound 4b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com