Macamides compound and synthetic method and application thereof
The technology of a macamide and a synthesis method, which is applied in the field of medicine, can solve the problem that a macamide monomer compound cannot be prepared in large quantities, and achieves the effects of low price, few by-products, and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
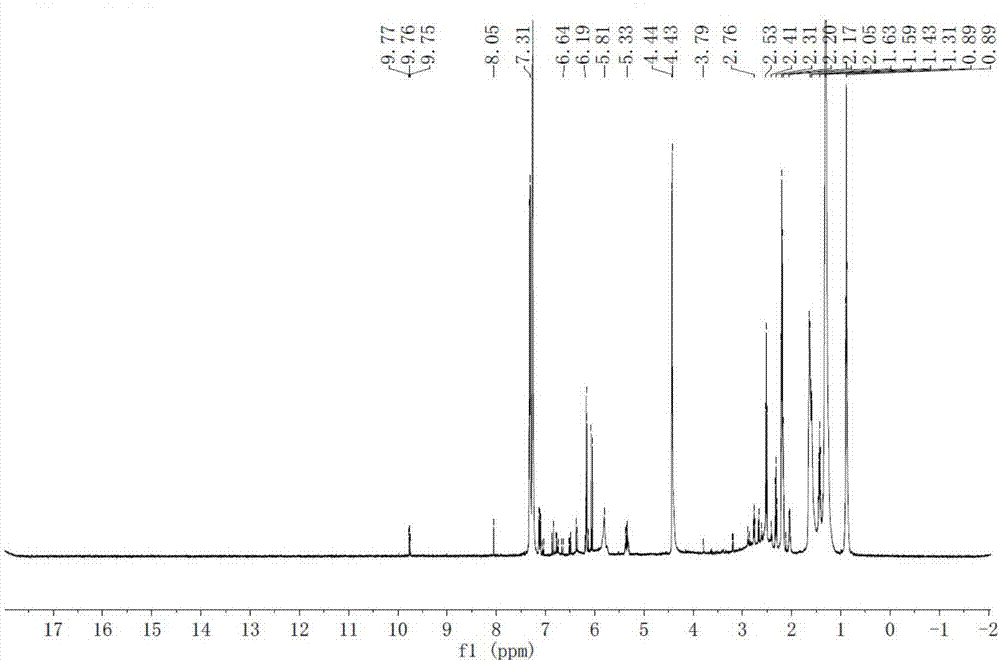
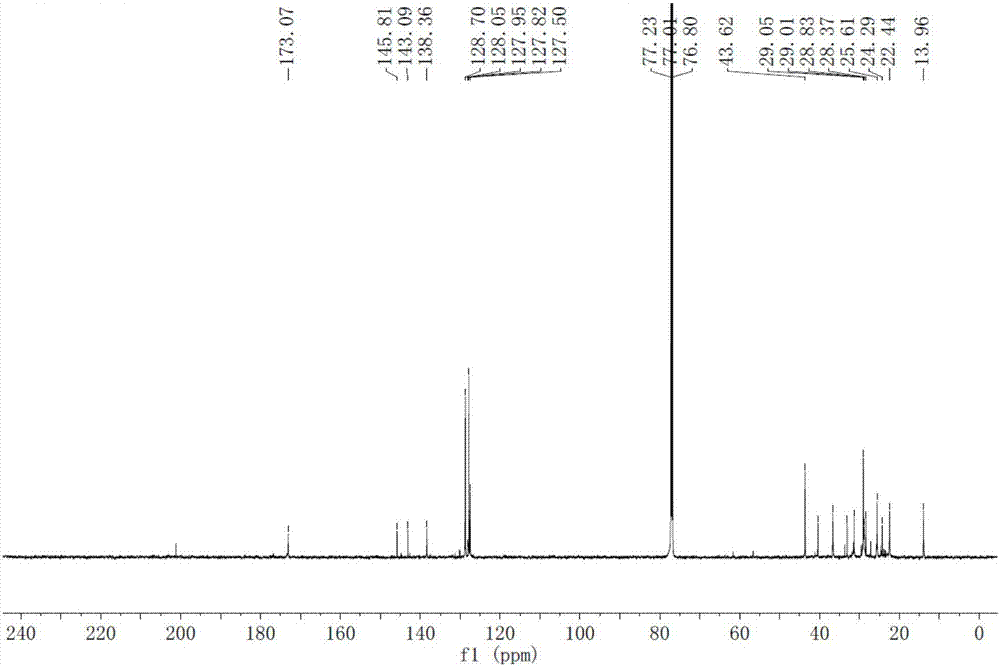
[0029] A method for synthesizing macamide, comprising: catalyzing and oxidizing linoleic acid and an oxidant to obtain a macaene mixture through pyridine derivatives; performing amidation reaction of the macaene mixture with benzylamine or benzylamine derivatives, and then separating by preparative chromatography;
[0030] Wherein: the oxidizing agent is any one of 2,2,6,6-tetramethylpiperidine-nitrogen-oxide, tribromopyridinium, and 2-iodylbenzoic acid. Benzylamine derivatives are 3-methoxybenzylamine or 3,4-dimethoxybenzylamine.
[0031] Linoleic acid, molecular formula: CH 3 (CH 2 ) 4 CH=CHCH 2 CH=CH(CH 2 ) 7 COOH; a kind of unsaturated fatty acid, colorless to pale yellow liquid, changes color when exposed to air.
[0032] TEMPO, tetramethylpiperidine nitrogen oxide; 2,2,6,6-tetramethylpiperidine-nitrogen-oxide; English name: 2,2,6,6-Tetramethyl-1-piperidinyloxy; Molecular formula: C 9 h 18 NO.
[0033] Py.HBr3 tribromopyridinium; English name: pyridinium hydrobro...
Embodiment 1
[0071] This embodiment provides a macamide compound, which is synthesized by the following steps:
[0072] Weigh 1g of linoleic acid and dissolve it in 20ml of acetonitrile, add 0.5g of 4-dimethylaminopyridine (DMAP) as a catalyst, then add 1.6g of TEMPO, react at room temperature for 4h, concentrate under reduced pressure at 30°C to remove acetonitrile, and wash the residue with 20ml of dimethicone Methane chloride was dissolved, and the filtrate was washed three times with saturated sodium carbonate solution and water successively, and concentrated under reduced pressure at 30°C to remove methylene chloride to obtain a macaene mixture.
[0073] Take 0.03mol of benzylamine and 0.02mol of the above-mentioned macaene mixture, add 50ml of dry dichloromethane, add 0.03mol of 4-dimethylaminopyridine, stir for 20min; then add 0.03mol of N,N'-cyclohexylcarbodiimide, The reaction was stirred at room temperature for 3 h, and filtered; the filtrate was washed with saturated sodium carb...
Embodiment 2
[0075] This embodiment provides a macamide compound, which is synthesized by the following steps:
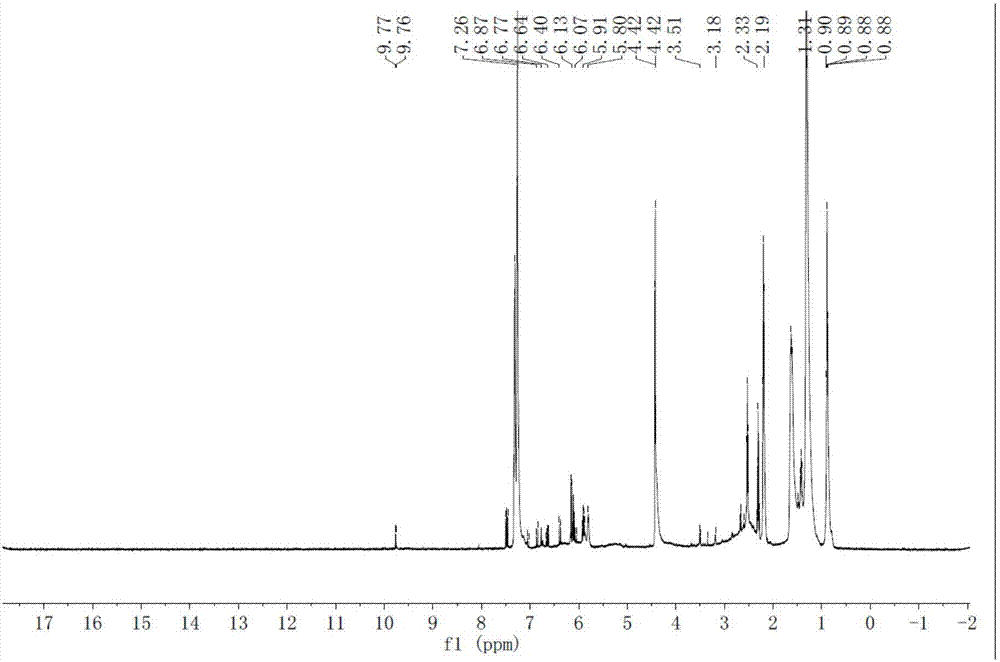
[0076] Weigh 1g of linoleic acid and dissolve it in 20ml of acetonitrile, add 0.5g of 4-pyrrolidinylpyridine as a catalyst, then add 1.6g of TEMPO, react at room temperature for 4h, concentrate under reduced pressure at 30°C to remove acetonitrile, and dissolve the residue in 20ml of dichloromethane. The filtrate was washed successively with saturated sodium carbonate solution and water three times, and concentrated under reduced pressure at 30°C to remove dichloromethane to obtain a macaene mixture.
[0077] Take 0.03mol 3,4-dimethoxybenzylamine and 0.02mol of the above-mentioned macaene mixture, add 50ml of dry dichloromethane, add 0.03mol 4-pyrrolidinylpyridine, stir for 20min; then add 0.03mol N, N' -Dicyclohexylcarbodiimide, stirred and reacted at room temperature for 3h, filtered; the filtrate was washed with saturated sodium carbonate solution and water three times in seq...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com