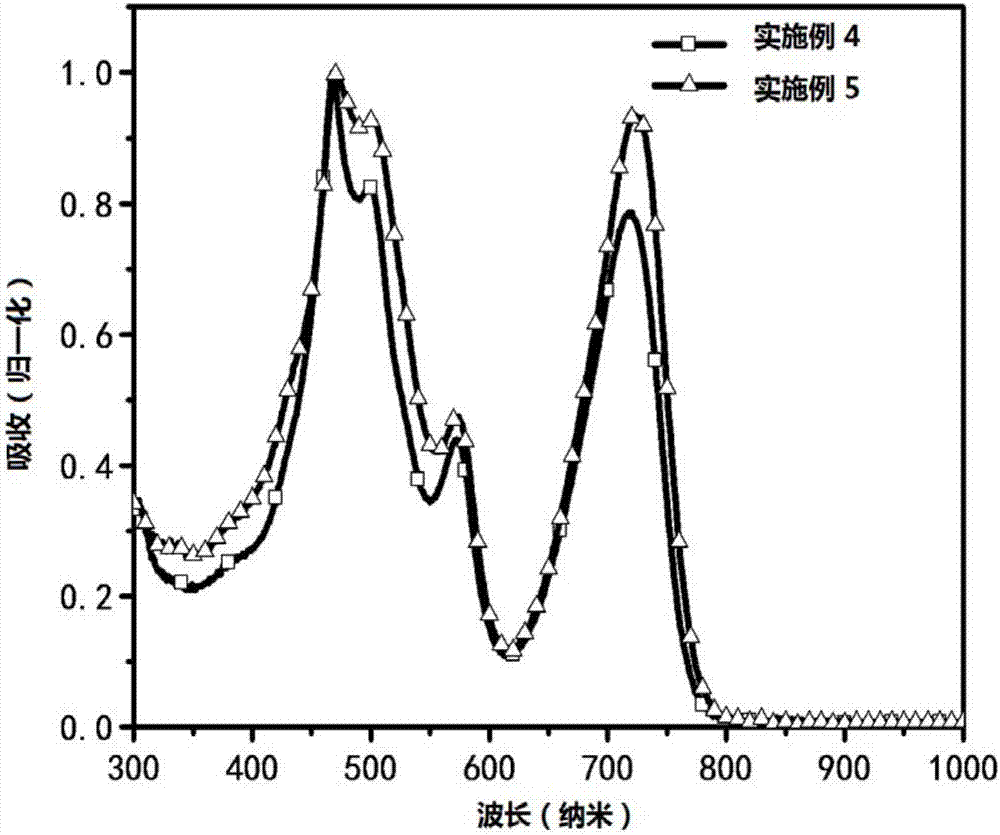
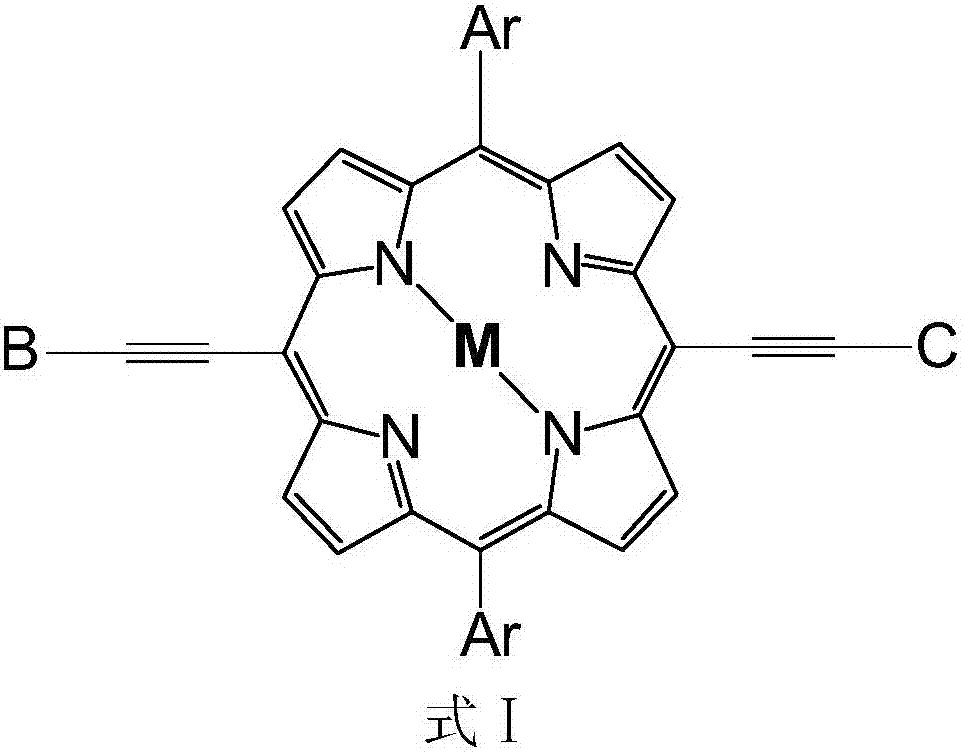
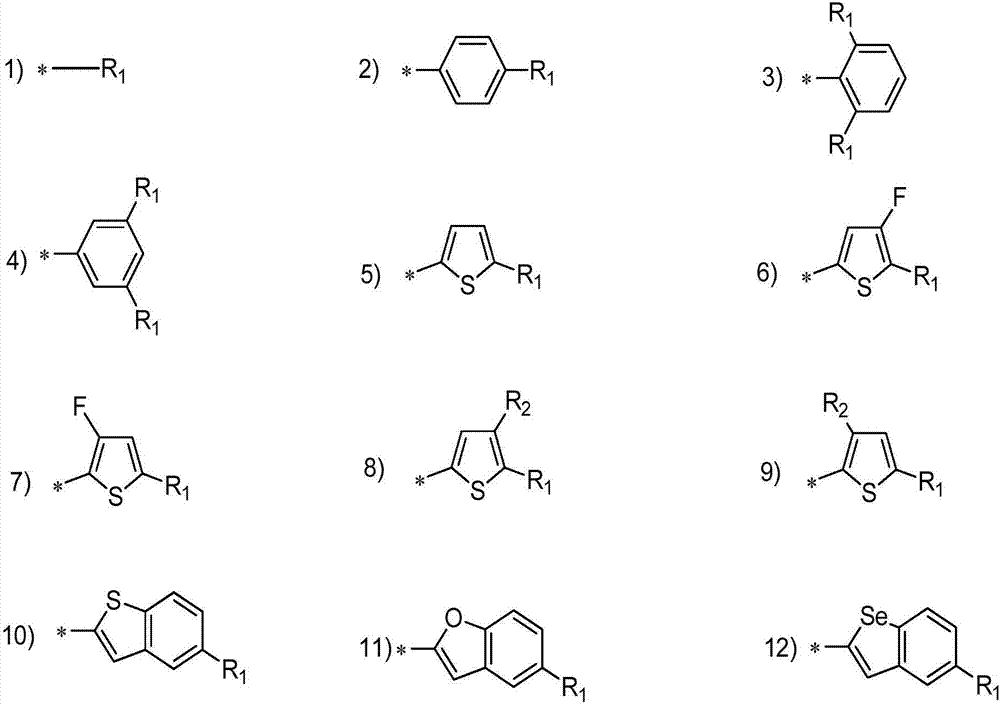
Asymmetric porphyrin organic micromolecule photovoltaic material as well as preparation method and application thereof
A photovoltaic material and small molecule technology, applied in photovoltaic power generation, organic chemistry, silicon organic compounds, etc., can solve the problem of low photoelectric conversion efficiency, and achieve the effects of optimizing solubility, wide spectral absorption range, and broadened absorption spectrum.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Synthesis of compound 3:
[0040] The equation for the reaction is as follows:
[0041]
[0042] Under the protection of argon, add 5-acetylene-15-(triisopropylsilylacetylene)-10,20-bis(5-(2-ethylhexyl)thiophene) zinc porphyrin into a 100mL two-neck round bottom flask Phenyl (compound 1) (762mg, 0.79mmol), 5-bromo-3-n-octyl-2-thiophene acetaldehyde (compound 2) (476mg, 1.58mmol), anhydrous THF (40mL), triethylamine (20mL ), Tetrakis(triphenylphosphine)palladium (90mg, 0.079mmol) and cuprous iodide (15mg, 0.079mmol), protected from light, stirred and reacted at 65°C for two days; after the reaction was completed, cooled to room temperature, washed with water, It was extracted with chloroform, dried over anhydrous sodium sulfate, and spin-dried, and the product was separated by silica gel column, and then gel permeation chromatography was used to obtain a purple solid, namely compound 3. 1H NMR (500MHz, deuterated chloroform) δ / ppm 9.85(s, 1H), 9.73(d, J=4.5Hz, 2H), ...
Embodiment 2
[0044] Synthesis of compound 4:
[0045] The equation for the reaction is as follows:
[0046]
[0047] Add compound 3 (680mg, 0.57mmol), THF (35mL) to a 100mL single-necked round-bottom flask, and then slowly add (1 drop / second) tetrabutylammonium fluoride (0.68mmol); after the reaction is complete, add water to quench , extracted with chloroform, dried over anhydrous sodium sulfate, and spin-dried to obtain a brown solid, namely compound 4, which was directly used in the next reaction after drying without further purification.
Embodiment 3
[0049] Synthesis of compound 6 (thienoporphyrin containing aldehyde group):
[0050] The equation for the reaction is as follows:
[0051]
[0052] Compound 4 (492mg, 0.47mmol) and 2,5-bis(2-ethylhexyl)-3,6-bis(2'-thienyl)-pyrrolo[3,4-c]pyrrole-1,4 (2H,5H)-Diketone (Compound 5) (425mg, 0.71mmol) was dissolved in 32mL of tetrahydrofuran and 16mL of triethylamine, then added tetrakis(triphenylphosphine) palladium (54mg, 0.047mmol) and iodide Cuprous (9 mg, 0.047 mmol), reacted at 65 ° C for 48 hours; after the reaction was completed, water was added to the reaction flask, extracted with chloroform, washed several times with water, and the organic phase was evaporated to dryness on a rotary evaporator to obtain the crude The product was purified by silica gel column, and compound 6 was obtained after vacuum drying. 1 H NMR (500 MHz, deuterated chloroform): δ9.85(s, 1H), 9.52(d, J=4.5Hz, 2H), 9.48(d, J=4.5Hz, 2H), 9.17(d, J= 4.5Hz, 4H), 8.92(d, J=4.5Hz, 1H), 8.72(m, 1H), 7.7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com