Candida antarctica lipase B mutant as well as transformation method and application thereof
A technology of Candida Antarctica and mutants, applied in the field of bioengineering, can solve problems such as hindering industrial application, poor enantioselectivity, and prolonging the production cycle, and achieve high-efficiency enantioselectivity and high enantioselectivity , The effect of reducing the production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Selection and screening of mutation sites
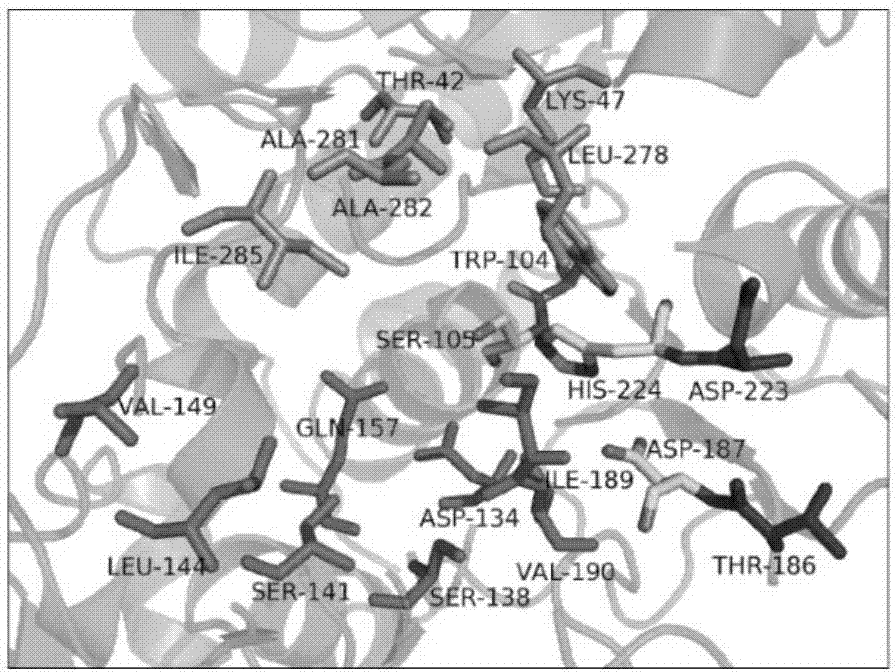
[0041] The active pocket of CALB is mainly composed of two parts: the acyl-binding pocket and the alcohol-binding pocket. The catalytic triad Asp187-His224-Ser105 is located in between. The acyl binding pocket is mainly composed of A141, L144, V149, D134, T138, and Q157, the alcohol binding pocket is mainly composed of T42, K47, W104, L278, A281, and A282, and A281, A282, and I285 are oriented toward the alcohol moiety of the substrate, limiting the size of the channel.
[0042] Selection of sites: Acyl pockets (D134, A148, V149, I189, V190, and Q157), alcohol pockets (T42, T43, W104, A281, and A282), A281 and A282 were taken into account because they are located at the channel; additionally , the proximity of D223 and T186 to the catalytic histidine, aspartic acid may have an effect on the conformation of the active pocket, taking into account, as figure 1 shown.
Embodiment 2
[0043] Example 2: Experimental verification of the effect of amino acid residues in simulated screening on enantioselectivity
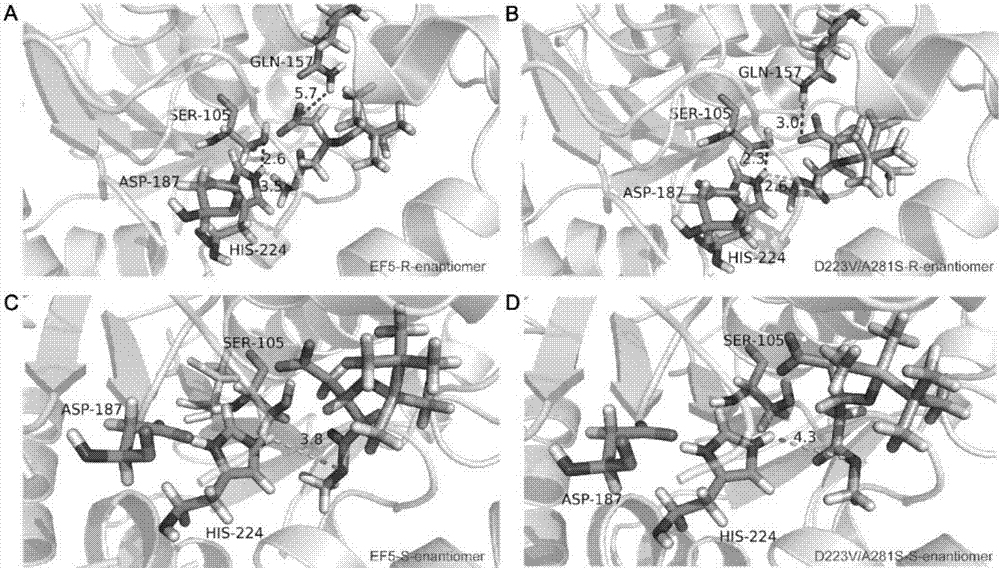
[0044] For the amino acid residues D134, A148, V149, I189, V190, Q157, T42, T43, W104, A281, A282, D223 and T186 in the simulation screening, the enantioselectivity was verified by constructing a mutation library and high-throughput screening Impact. Combine the above mutation sites into library 1 (A148 / V149), library 2 (I189 / V190), library 3 (Q157), library 4 (T42 / T43), library 5 (W104), library 6 (A281 / A282) , library 7 (D223), library 8 (T186), library 9 (D134), construct the above nine mutant libraries, and through high-throughput screening, screen all mutants in all mutant libraries to verify enantiomeric selection Sexual changes. After screening nearly 7,000 mutants, according to the screening results, only D223V, A281S and D223V / A281S had significant changes in enantioselectivity, and A282S, W104A, and Q157N were selected for experimental ver...
Embodiment 3
[0045] Embodiment 3: the construction of mutant
[0046] Six mutants D223V, A281S, A282S, W104A, Q157N and D223V / A281S were successfully constructed by site-directed mutagenesis. First, use the plasmid connected with the parental EF5 gene on the T vector as a template, respectively with SEQ ID NO.5 and SEQ ID NO.6, SEQ ID NO.7 and SEQ ID NO.8, SEQ ID NO.9 and SEQ ID NO .10, SEQ ID NO.11 and SEQ ID NO.12, SEQ ID NO.13 and SEQ ID NO.14 are primers, utilize high-fidelity enzyme PrimerStar to amplify, utilize restriction endonuclease XhoI and XbaI double enzyme digestion (37 °C, 3h), the mutant gene was recovered. The obtained mutant gene was ligated with the PGAPZαA expression vector recovered by the same endonuclease digestion at 16°C overnight, and the ligated product was transformed into JM109 competent cells for amplification. The recombinant plasmid was extracted, linearized with endonuclease AVrII (37°C, 3h), recovered from the column, electrotransformed into GS115 compet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com