Anti-tumor metal iridium (III) complex as well as preparation method and application thereof
An anti-tumor drug, metal iridium technology, applied in the direction of anti-tumor drugs, indium organic compounds, platinum group organic compounds, etc., can solve the problems of interaction, difficulty, short remission period, etc., and achieve inhibition of tumor growth and high yield , the effect of strong tumor growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
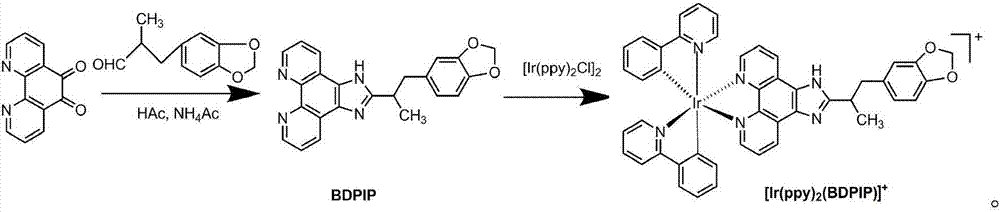
[0030] Synthetic BDPIP ligand
[0031] 1.5mmol (0.315g) of 1,10-phenanthroline-5,6-dione and 1.5mmol (0.165g) of 2-methyl-3-(3,4-methylenedioxyphenylcyclo)propane Add aldehyde to the reaction vessel, add 30mmol (2.31g) ammonium acetate and 20mL glacial acetic acid solvent, heat to reflux for 2 hours, cool, adjust the pH value to close to 7.0 with ammonia water, filter with suction to obtain a light red solid, wash with 30mL distilled water three times, The BDPIP ligand is obtained, and then the obtained BDPIP ligand is dried in a vacuum oven.
[0032] Yield: 76%.Anal.Calcd for C 23 h 18 N 4 o 2 : C, 72.22; H, 4.75; N, 14.66.Found: C, 72.35; H, 4.91; N, 14.53%.IR(KBr, cm -1 ):3434b, 2972m, 1622s, 1566w, 1542w, 1502w, 1489w, 1442m, 1410w, 1355m, 1281m, 1248s, 1190m, 1125m, 1099m, 1064m, 1038s, 929s, 808s, 739s.ESI=-MS:m / z 383[M+1].
[0033] The main synthesis route of the above-mentioned process is as follows:
[0034]
Embodiment 2
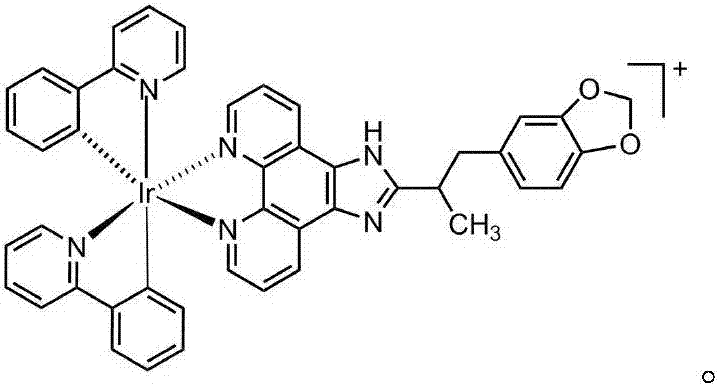
[0036] In this experiment, the iridium metal complex [Ir(ppy) 2 (BDPIP)]PF 6
[0037] Complexes [Ir(ppy) 2 (BDPIP)]PF 6 Synthesis of: [Ir(ppy) 2 Cl] 2 Mix with BDPIP ligand at a molar ratio of 1:2, in a mixed solvent of 42mL dichloromethane and methanol (2:1 by volume), reflux under argon protection for 6 hours, cool to room temperature, add NH 4 PF 6 The solution was then stirred at room temperature for 2 hours to obtain a red precipitate, which was filtered under reduced pressure to obtain a large amount of solid. The obtained solid mixture was dissolved in dichloromethane, suction-filtered, and the insoluble matter was filtered off to obtain a yellow liquid, which was then rotary evaporated under reduced pressure to obtain an orange-yellow solid.
[0038] Yield: 72%. Anal. Calc for C 45 h 34 N 6 o 2 IrPF 6 : C, 52.52; H, 3.33; N, 8.17%.Found: C, 52.68; H, 3.51; N, 8.23%.IR(KBr, cm -1 ):3399w, 3137s, 1625m, 1608m, 1584w, 1502w, 1479s, 1402s, 1316w, 1282w, 1268w...
Embodiment 3
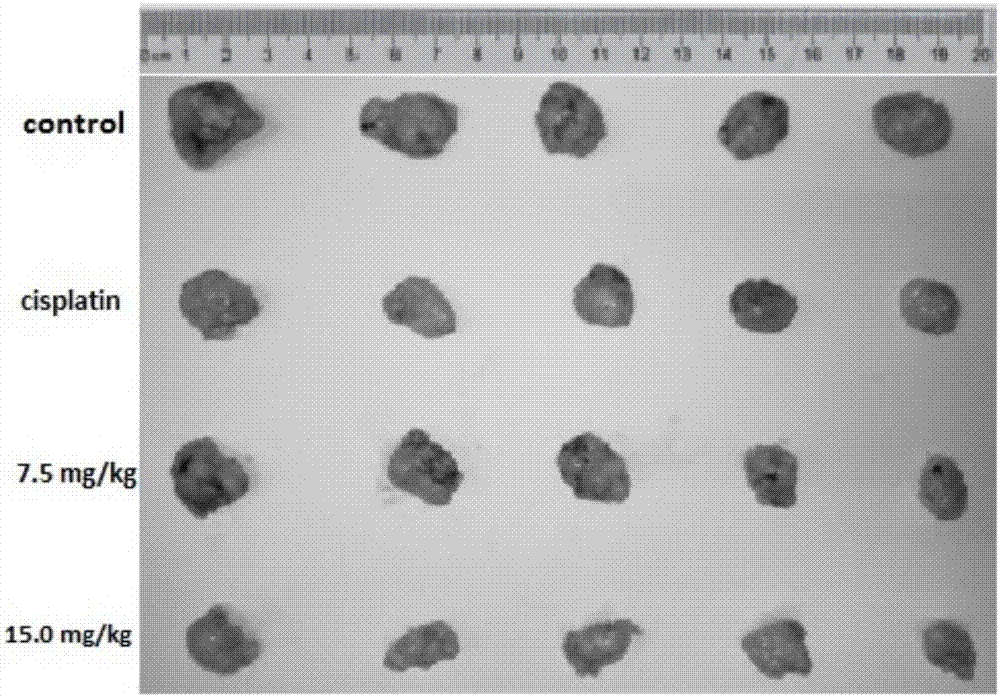
[0042] The present embodiment detects the iridium metal complex [Ir(ppy) by experiment 2 (BDPIP)]PF 6 Inhibitory effect on proliferation of tumor cell A549
[0043] Cytotoxicity test: In this example, the in vitro cytotoxicity of the compounds was studied by the MTT method. First, trypsinize the target cells cultured into a single layer, count them with a cell counter, and add appropriate RPMI-1640 to make the number of cells reach 10 5 For each milliliter, take 0.1mL of cell-containing culture solution in a 96-well cell culture plate, and then place the inoculated cell plate at 37°C, 5% CO 2 cultured in an incubator for 24 h. Add different concentrations of compounds to the discarded liquid of the monolayer cell culture plate, and the concentration of the test compound reaches 10 -6 to 10 -4 M, and set up a control group (0.1mL medium was added to the cell culture plate), placed in 37°C, 5% CO 2 After culturing in the incubator for 48 hours, the culture medium was aspir...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com