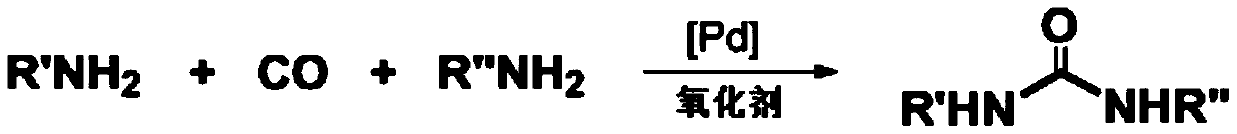A kind of method of catalytic oxidative carbonylation synthesis unsymmetrical disubstituted urea
A disubstituted urea, catalytic oxidation technology, applied in the direction of organic chemical methods, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of poor selectivity of disubstituted urea, difficult product post-processing, high toxicity of raw materials, etc., to achieve selective High reactivity, wide range of oxidant sources, simple and easy synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
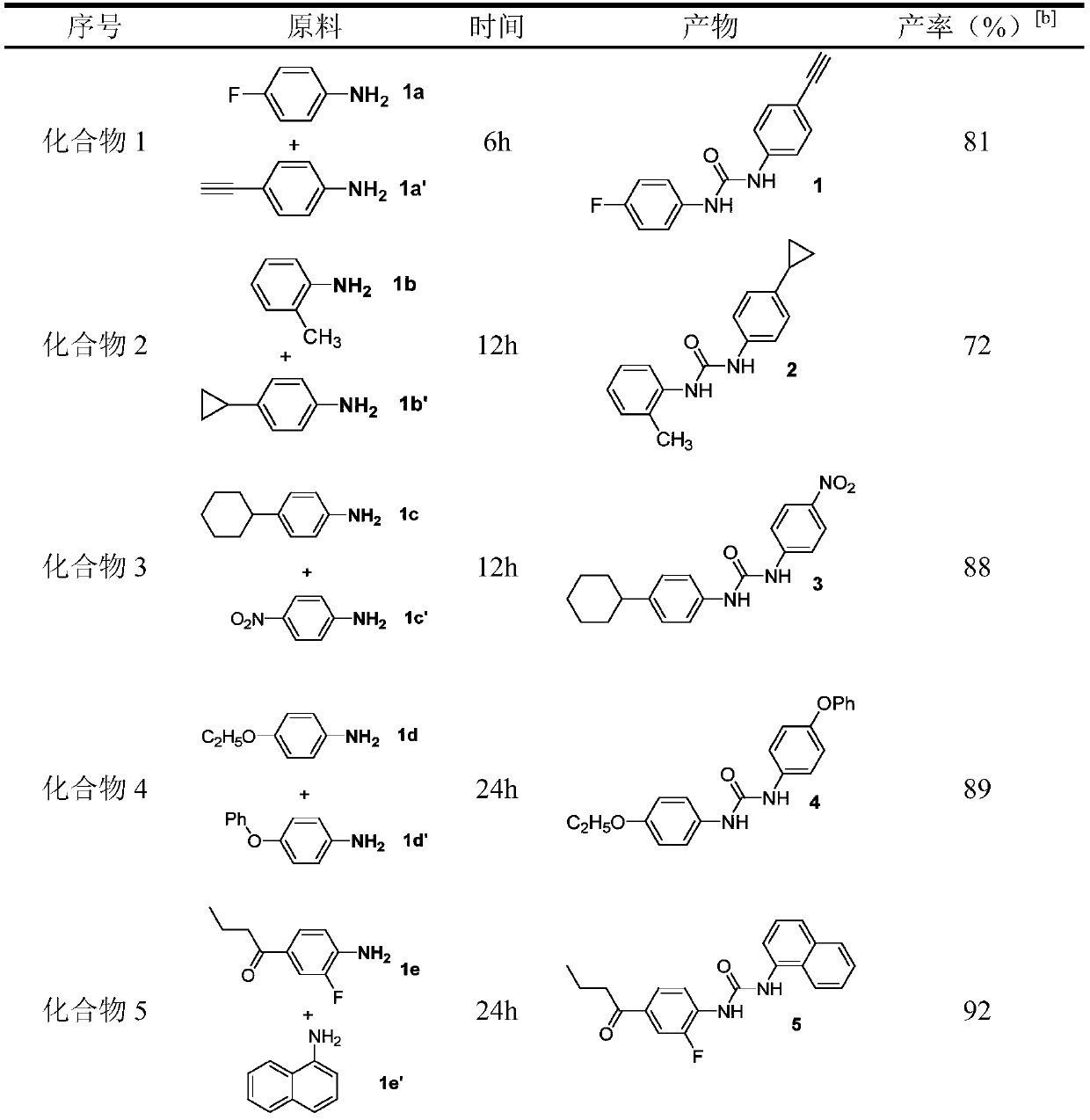
[0034] Compound 1: Palladium acetate (0.005mmol), amine 1a (0.5mmol), amine 1a' (0.75mmol), triethylamine (0.1mmol), sodium iodide (0.25mmol) and polyethylene glycol were sequentially added to a 25mL reaction flask. Alcohol-400 (2.0g), and introduce an atmospheric pressure of carbon monoxide and oxygen (1:1), react at 25°C for 6h. Cool to room temperature, extract, evaporate the solvent under reduced pressure, and separate by column chromatography to obtain a yield of 81%.
Embodiment 2
[0036]Compound 2: Add palladium chloride (0.005mmol), amine 1b (0.5mmol), amine 1b' (1.0mmol), potassium phosphate (0.1mmol), potassium iodide (0.25mmol) and polyethylene glycol- 400 (2.0g), and introduce an atmospheric pressure of carbon monoxide and oxygen (1:1), and react at 80°C for 12h. Cool to room temperature, extract, evaporate the solvent under reduced pressure, and separate by column chromatography to obtain a yield of 72%.
Embodiment 3
[0038] Compound 3: Add palladium carbon (0.01mmol), amine 1c (0.5mmol), amine 1c' (1.0mmol), triethylenediamine (0.1mmol), sodium iodide (0.25mmol) and polyethylene Diol-600 (2.0g) was introduced into an atmospheric pressure of carbon monoxide and oxygen (1:1), and reacted at 80°C for 12h. Cool to room temperature, extract, evaporate the solvent under reduced pressure, and separate by column chromatography to obtain a yield of 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com