Synthesis method of musk ambrette
A synthesis method and technology of musk sunflower, applied in the field of essence, can solve the problems of low production efficiency and large amount of waste water, and achieve the effects of reducing energy consumption and saving human resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
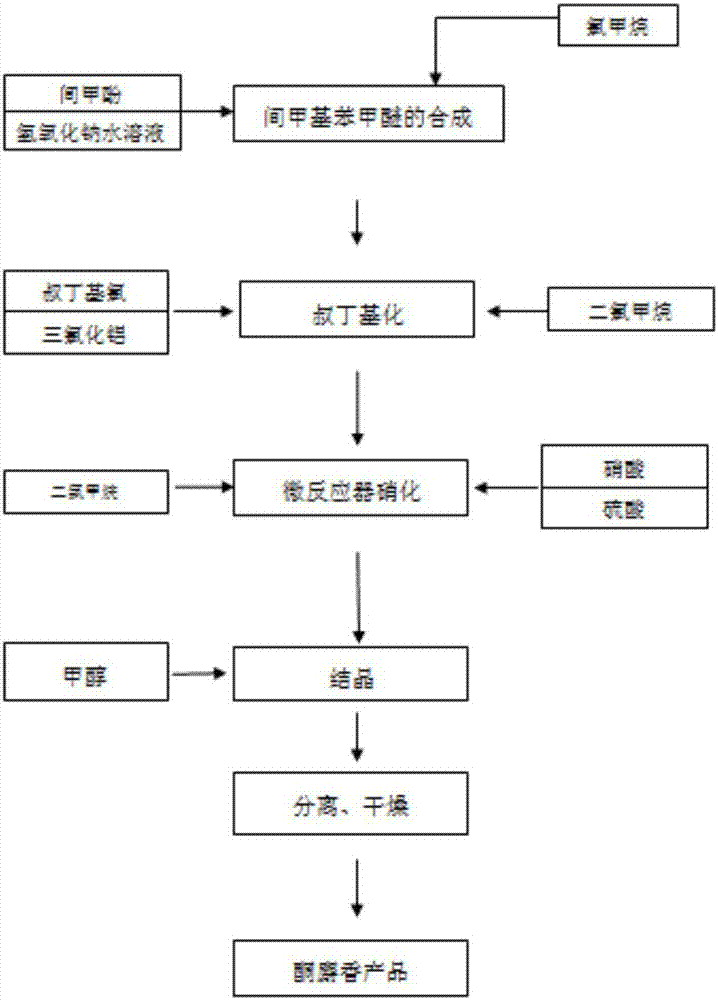
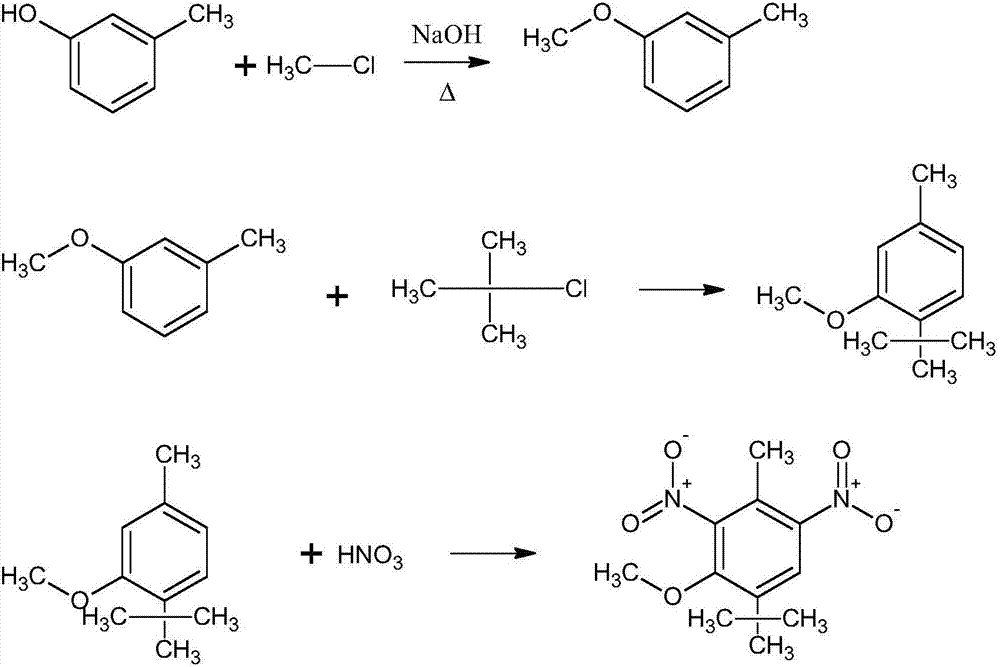
[0028] The synthetic method of described sunflower musk, comprises the steps:
[0029] (1) Add 108g (0.99mol, 99%) m-cresol, 355g20% sodium hydroxide (1.775mol) aqueous solution, 4.3g (0.023mol, 99%) benzyltrimethylammonium chloride in 1000ml autoclave , 60.5g (1.186mol, 99%) methyl chloride carries out methoxylation reaction, is warming up to 80 ℃, is incubated 6-8 hour, sampling analysis selectivity 96.2%, then separates organic phase and neutralizes with 35% hydrochloric acid, after After washing and rectification under reduced pressure, 113.3 g of m-methylanisole was obtained, with a content of 98.5%, and a molar yield of 91.6%. The reason for affecting the yield was that the high concentration of sodium hydroxide formed a side reaction polymer;
[0030] (2) In four bottles of 1000ml equipped with reflux condenser, constant pressure dropping funnel, and chlorine trap, add 122g (1.42mol, 99%) of methylene chloride, 56g (0.41mol, 98%) of anhydrous aluminum trichloride %), t...
Embodiment 2
[0033] The synthetic method of described sunflower musk, comprises the steps:
[0034] (1) in 1000ml autoclave, add 108g (0.99mol, 99%) m-cresol, 355g15% (1.33mol) sodium hydroxide aqueous solution, 4.3g (0.023mol, 99%) benzyl trimethyl ammonium chloride, 60.5g (1.186mol, 99%) methyl chloride, warming up to 100°C and insulated for 6-8 hours to carry out methoxylation reaction, sampling analysis selectivity 97.1%, then separate the organic phase and neutralize with 35% hydrochloric acid, after washing, After rectification under reduced pressure, 112.5 g of m-methylanisole was obtained, with a content of 98.3% and a molar yield of 90.8%. The main reason for affecting the yield was that the high temperature formed side reaction polymerization;
[0035] (2) In four bottles of 1000ml equipped with a reflux condenser, a constant pressure dropping funnel, and a chlorine trap, add 122g (1.42mol, 99%) of methylene chloride, 41g (0.3mol, 98%) of anhydrous aluminum trichloride %), contr...
Embodiment 3
[0038] The synthetic method of described sunflower musk, comprises the steps:
[0039](1) in 1000ml autoclave, add 108g (0.99mol, 99%) m-cresol, 355g15% sodium hydroxide (1.33mol) aqueous solution, 4.3g (0.023mol, 99%) benzyl trimethyl ammonium chloride, 60.5g (1.186mol, 99%) of methyl chloride was heated to 70°C and kept for 6-8 hours, and the selectivity of sampling analysis was 94.8%, then the organic phase was separated and neutralized with 35% hydrochloric acid, washed and rectified under reduced pressure to obtain m-methylanisole 113.5g, content 99%, molar yield 92.2%, the main reason for affecting the yield is the incomplete reaction of raw material m-cresol at low temperature;
[0040] (2) In four bottles of 1000ml equipped with a reflux condenser, a constant pressure dropping funnel, and a chlorine trap, add 122g (1.42mol, 99%) of methylene chloride, 34g (0.025mol, 98%) of anhydrous aluminum trichloride %), control the temperature in the bottle at 0-5°C and add 123.2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com