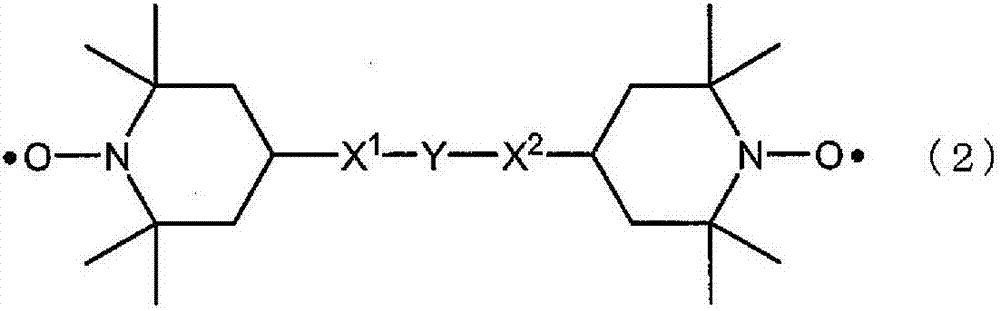
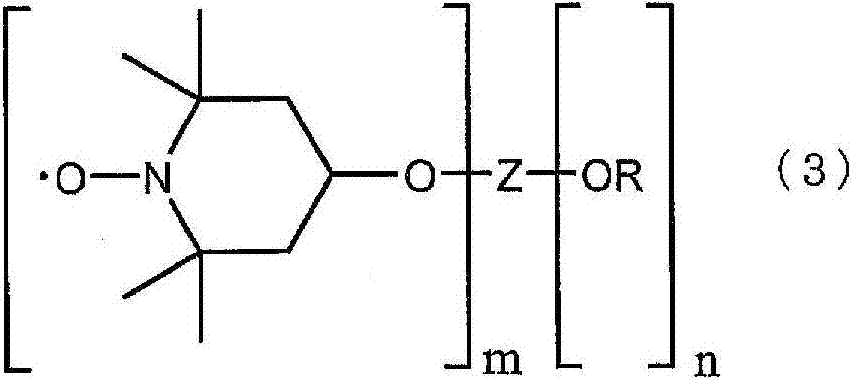
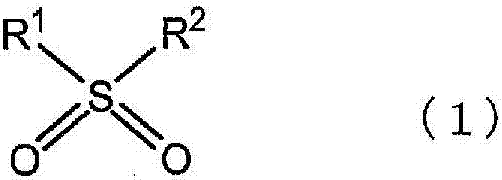Dye-sensitized solar cell and electrolysis solution for dye-sensitized solar cell
A technology for sensitization of solar cells and pigments, applied in the field of electrolytes, can solve the problems of decreased diffusivity of charge transport materials, decreased battery characteristics, and high volatility, achieve long-term stabilization of battery characteristics, and contribute to durability and thermal stability. high sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example 1
[0117] [Production Example 1] Synthesis of bis(2,2,6,6-tetramethyl-4-piperidinyl-1-oxy) sebacate (compound (A))
[0118] [Chemical formula 13]
[0119]
[0120] A 200mL 4-necked flask equipped with a stirrer, a nitrogen introduction tube, a thermometer, and a reflux condenser was fully vented with nitrogen, and then 50 mL of tetrahydrofuran, 1.11 g (0.011 mol) of triethylamine, and 4-hydroxy-2,2,6 ,6-Tetramethylpiperidine (manufactured by Tokyo Chemical Industry Co., Ltd., trade name: TEMPOL) 1.72 g (0.010 mol) to obtain a homogeneous solution. Next, after cooling the 4-necked flask to 10°C or lower with ice water, 20 mL of a tetrahydrofuran solution containing 1.01 g (0.005 mol) of sebacic acid (manufactured by Tokyo Chemical Industry Co., Ltd.) and 1.31 g (0.011 mol) of thionyl chloride was added. Continuously drip into the homogeneous solution. After the dropping, the reaction was performed for 2 hours while keeping the reaction liquid at 10°C or lower, and then the reaction w...
manufacture example 2
[0122] Synthesis of bis(6-(2,2,6,6-tetramethyl-4-piperidinyl-1-oxy)hexyl) sebacate (compound (B))
[0123] [Chemical formula 14]
[0124]
manufacture example 2-1
[0125] (Production Example 2-1) Intermediate (B1): Synthesis of ethyl (2,2,6,6-tetramethyl-4-piperidylidene) acetate
[0126] After fully ventilating nitrogen in a 500 mL 4-necked flask equipped with a stirrer, nitrogen introduction tube, thermometer, and reflux condenser, 4.0 g (0.10 mol) of sodium hydride and 100 mL of diethyl ether were mixed, and the phosphono group was added slowly 25.1 g (0.11 mole) of triethyl acetate. Next, 80 mL of a diethyl ether solution of 10.9 g (0.07 mol) of 2,2,6,6-tetramethyl-4-piperidone was slowly added, and the reaction was continued for 15 hours. After that, water was added, extraction was repeated several times with diethyl ether, and after dehydration with magnesium sulfate, the solvent was removed with an evaporator to obtain 8.7 g (0.039 mol) of intermediate (B1). In addition, the molecular weight of the obtained product measured by mass analysis was 225, and it was confirmed that ethyl (2,2,6,6-tetramethyl-4-piperidide) acetate (intermed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com