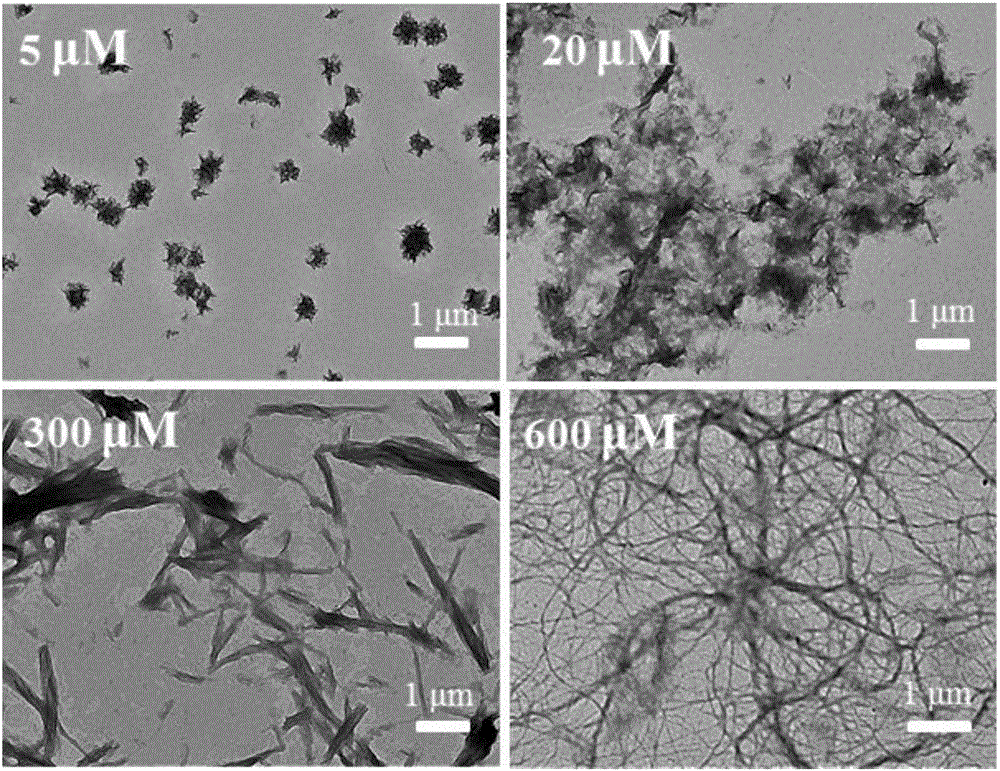
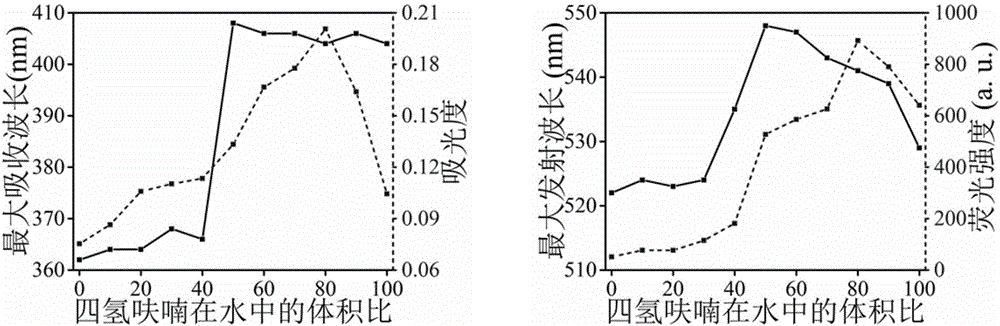
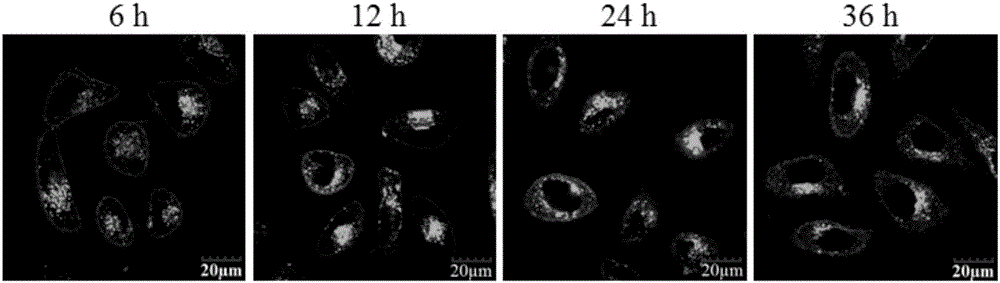
Modified conjugated polymer, and preparation method and application thereof
A conjugated polymer and modified technology, applied in the field of biomedicine, can solve the problems of unstable cell membrane structure binding and poor biocompatibility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The second aspect of the present invention provides the preparation method of the above-mentioned modified conjugated polymer, the method comprises: under the condition that solvent and catalyst exist, the conjugated polymer and the compound shown in formula (III) or formula (IV) Carry out click chemistry synthesis reaction,
[0038]
[0039] Among them, R 4 for C 2 -C 20 straight-chain alkyne group.
[0040] In a specific embodiment of the present invention, the R 4 Can be but not limited to: propynyl, butynyl or pentynyl. In preferred cases, the R 4 For butynyl.
[0041] In a specific embodiment of the present invention, in the presence of a solvent and a catalyst, the conjugated polymer and the compound represented by formula (III) are subjected to click chemical synthesis to prepare the modified compound represented by formula (I). Conjugated polymers.
[0042] In another specific embodiment of the present invention, in the presence of a solvent and a cat...
Embodiment 1
[0071] (1) Preparation of conjugated polymer
[0072] (1a) Synthesis of (E)-1-bromo-4-(4-bromoethylstyryl)benzene
[0073] Diethyl 4-bromobenzylphosphonate (purchased from Bailingwei Company, batch number 377896) (2.0g, 6.5mmol), sodium tert-butoxide (NaO t Bu) (0.69g, 7.2mmol) and 40mL of anhydrous tetrahydrofuran (THF) were successively added into a 100mL Schlenk flask, after alternate freezing, evacuation and thawing and degassing for 3 times, the solution containing 4-(methyl bromide) was added dropwise. (1.29 g, 6.5 mmol) in 10 mL of degassed and dried THF, and the above mixture was kept stirring at 0° C. for 30 minutes. After removing the ice bath, the reaction mixture was stirred at room temperature for 24 hours. The reaction was quenched with water and extracted with dichloromethane. Subsequently, the organic layer was washed with brine, washed with Na 2 SO 4 Dry and spin dry the organic phase. The crude product was purified by column chromatography (petroleum et...
Embodiment 2
[0142] (1) Preparation of conjugated polymer
[0143] (1a) 9,9-bis(6′-bromohexyl)-2,7-dibromofluorene
[0144] 1,6-dibromohexane (97.6g, 400mmol) was added in 100ml of 50% potassium hydroxide solution, 1.28g of phase transfer catalyst tetrabutylammonium bromide (TBAB) was added, and the temperature of the reaction solution was raised to 75°C , then added 2,7-dibromofluorene (12.96 g, 40 mmol) and stirred for 15 min. After the reaction was stopped and cooled to room temperature, dichloromethane extracted (100ml, performed 3 times), the organic phases were combined, washed with 1M hydrochloric acid solution and distilled water, dried over anhydrous magnesium sulfate, filtered and concentrated. The crude product was separated and purified by silica gel column chromatography, and the developing solvent was dichloromethane / petroleum ether (v / v=1 / 9) to obtain product V as a white solid (mass 21.4 g, yield 82%).
[0145] The hydrogen spectrum data of V is: 1 H-NMR: (300MHz, CDCl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com