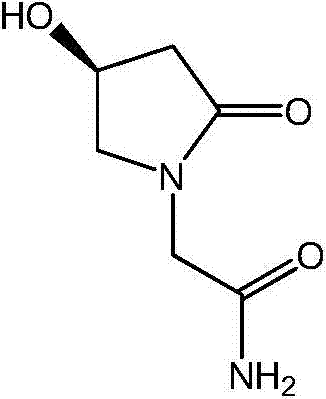
L-oxiracetam injection and preparation method thereof
An injection and injection technology, applied in the field of levooxiracetam, can solve the problems of lack of sterility and cannot guarantee the clinical safety of levooxiracetam injection, and achieve stable product quality, guaranteed effectiveness and high quality. The effect of safety and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Levoxiracetam injection is prepared according to the following steps:
[0042] Element
Dosage
Levoxiracetam
100g
0.3g
0.3g
Thimerosal
0.2g
Sterile water for injection
Add to 1000ml
[0043] 1. Concentrated formulation: Dissolve calcium sodium edetate, ascorbic acid and thimerosal with sterile water for injection of 50%-70% of the prescription amount, then add levoxiracetam and stir to dissolve;
[0044] 2. Dilute formulation: take the concentrated formulation, add citric acid-sodium citrate solution (citrate ion concentration is 30mmol / L), adjust the pH to 5.5, stir, mix well, add sterile water for injection to the prescribed amount;
[0045] 3. Potting and sealing: use 0.22μm acetate membrane material microporous membrane to filter, fill and seal;
[0046] 4. Sterilization: Send the filled ampoule into a steam sterilizer for sterilization at 121°C for 15 minutes. S...
Embodiment 2
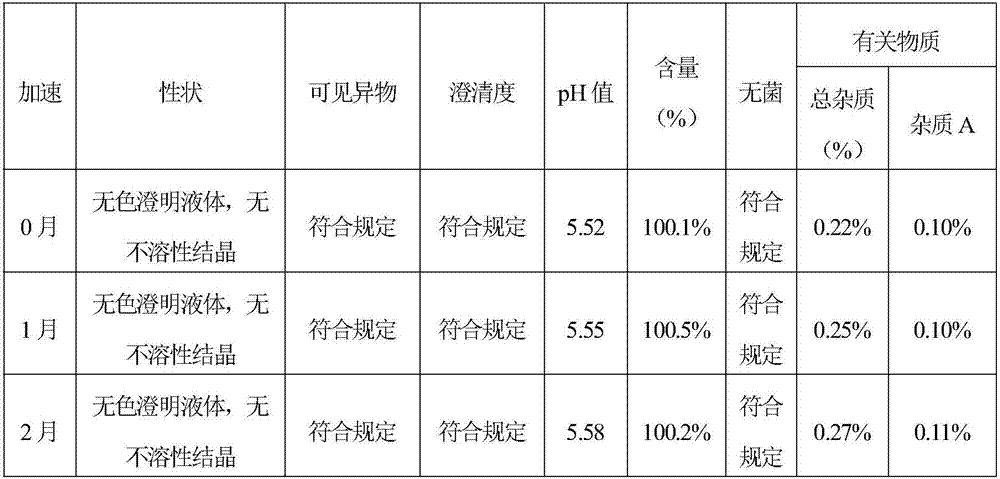
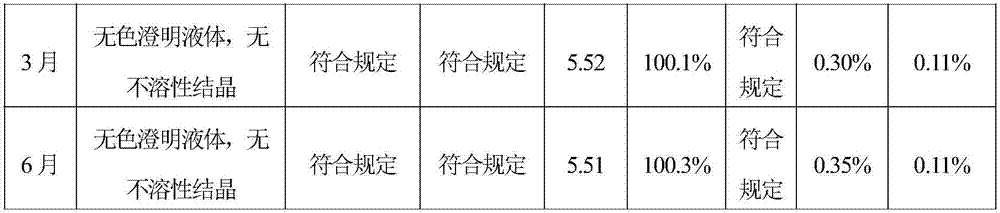
[0049] Stability Test of Levoxiracetam Injection
[0050] Experimental Materials:
[0051] Oxiracetam sample for injection: prepared for Example 1.
[0052] Accelerated test method: the oxiracetam for injection prepared in Example 1 is packaged according to the market, placed in an accelerated test box, and samples are taken for a certain period of time, and the investigation items are inspected.
[0053] Accelerated experiment temperature: 40±2℃
[0054] Humidity: RH75%±5%
[0055] Inspection time: 0, 1, 2, 3, 6 months
[0056] Inspection indicators: traits, visible foreign matter, clarity, pH, related substances (impurity A is S-4 hydroxy-2-oxo-1-pyrrolidine acetic acid), content, sterility test
[0057] Accelerated test stability records:
[0058]
[0059]
[0060] The results of the accelerated test show that the quality of the test indicators of the sample in June and the sample in 0 month is equivalent, indicating that the quality of this product remains stabl...
Embodiment 3
[0070] Levoxiracetam injection is prepared according to the following steps:
[0071] Element
Dosage
Levoxiracetam
60g
0.2g
ascorbic acid
0.2g
Thimerosal
0.2g
Sterile water for injection
Add to 1000ml
[0072] Preparation process:
[0073] 1. Concentrated formulation: Dissolve calcium sodium edetate, ascorbic acid and thimerosal with sterile water for injection of 50%-70% of the prescription amount, then add levoxiracetam and stir to dissolve;
[0074] 2. Dilute formulation: take the concentrated formulation, add citric acid-sodium citrate solution (citrate ion concentration is 15mmol / L), adjust the pH to 4.8, stir, mix well, add sterile water for injection to the prescribed amount;
[0075] 3. Potting and sealing: use 0.22μm acetate membrane material microporous membrane to filter, fill and seal;
[0076] 4. Sterilization: Send the filled ampoule into a steam sterilizer for sterilizat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com