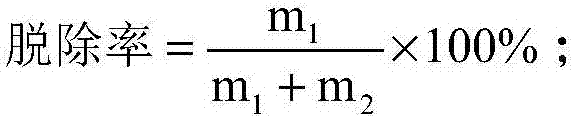Method for recycling lithium, nickel, cobalt and manganese from waste lithium ion battery
A lithium-ion battery and lithium recovery technology, which is applied in the field of nickel, lithium recovery, cobalt, and manganese, can solve the problems of easy generation of toxic gases, etc., and achieve the effect of high extraction rate and high removal rate in the later stage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
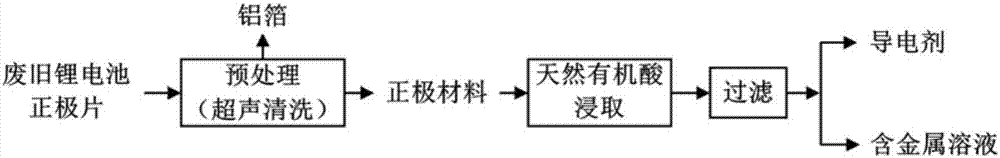
[0063] Such as figure 1 As shown, the recovery route is as follows:
[0064] (1) Preprocessing:
[0065] Fully discharge the used lithium-ion battery, physically disassemble and remove the shell in the glove box, and take out the positive electrode piece; cut the positive electrode piece into a size of 2cm×2cm, and put it into the conical flask; add N,N-2 After methyl acetamide, put it into an ultrasonic cleaning machine, control the temperature at 60°C, and ultrasonic frequency at 20KHz, and wash for 30 minutes; filter, the separated organic solvent is recycled, and the solid matter is dried and passed through a 50 μm sieve. The current collector is aluminum foil, and the undersize is positive electrode active material and conductive agent. In this example, the removal rate of the positive electrode active material was 71.71%.
[0066] (2) Leach lithium, nickel, cobalt, manganese:
[0067] After the positive electrode material (active material and conductive agent) is com...
Embodiment 2
[0075] (1) Preprocessing:
[0076] Fully discharge the used lithium-ion battery, physically disassemble and remove the shell in the glove box, and take out the positive electrode piece; cut the positive electrode piece into a size of 2cm×2cm, and put it into the conical flask; add N,N-2 After methyl formamide, put it into an ultrasonic cleaning machine, control the temperature at 60°C, and the ultrasonic frequency at 40KHz, and wash for 30 minutes; filter, the separated solvent is recycled, and the solid matter is dried and passed through a 50 μm sieve. Fluid aluminum foil, the undersize is positive electrode active material and conductive agent. In this example, the removal rate of positive electrode active material is 62.11%.
[0077] (2) Leach lithium, nickel, cobalt, manganese:
[0078] After the positive electrode material is completely dried, a mixed solution composed of 1.5M succinic acid and 0.15M sodium bisulfite is added to carry out leaching reaction. The reactio...
Embodiment 3
[0080] (1) Preprocessing:
[0081] Fully discharge the used lithium-ion battery, physically disassemble and remove the shell in the glove box, and take out the positive electrode piece; cut the positive electrode piece into a size of 2cm×2cm, and put it into the Erlenmeyer flask; add ethanol to the Erlenmeyer flask, put it Put it into an ultrasonic cleaning machine, control the temperature at 70°C, and the ultrasonic frequency at 40KHz, and wash for 30 minutes; filter, the separated solvent is recycled, and the solid matter is dried and passed through a 50 μm sieve. Active material and conductive agent. In this example, the removal rate of positive electrode active material is 6.21%.
[0082] (2) Leach lithium, nickel, cobalt, manganese:
[0083] After the positive electrode material is completely dried, a mixed solution composed of 1M tartaric acid and 1.28M hydrogen peroxide is added for leaching reaction. The reaction temperature is 70°C, the time is 0.5h, and the solid-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com