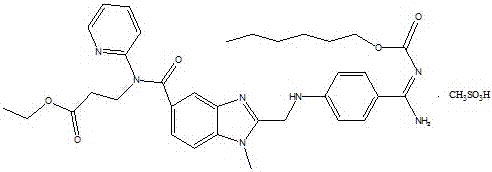
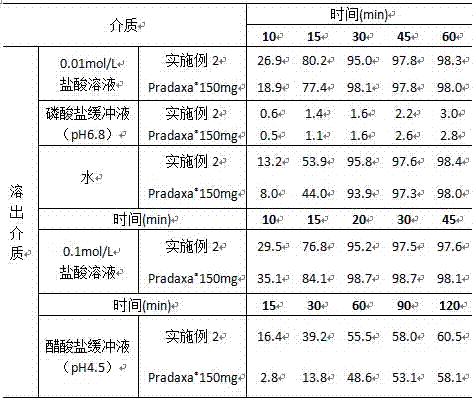
Stable capsule preparation containing dabigatran etexilate mesylate and preparation method of stable capsule preparation
A technology of dabigatran etexilate mesylate and tartaric acid is applied in the field of pharmaceutical preparations, which can solve the problems of rupture of the isolation membrane and reduce the stability of drugs, and achieve the effects of good dissolution, ensuring stability and product stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
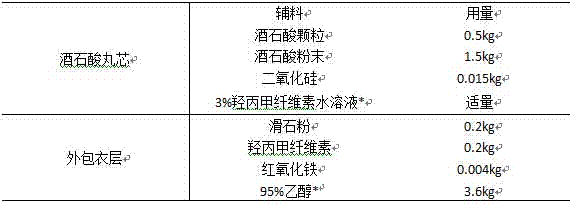
[0060] Preparation of tartrate pellets
[0061]
[0062] *Water and 95% ethanol are solvents that are removed during the process.
[0063] Preparation Process:
[0064] (1) Ball core process: Put the prescribed amount of tartaric acid granules (30-40 mesh) into the centrifugal granulation coating machine, mix the tartaric acid powder (100 mesh) and silicon dioxide, put them into the powder supply machine, and turn on The main engine and the blower can be adjusted until the particle flow in the cavity is smooth. Set the material temperature to 40°C, and when the temperature of the granules reaches the required temperature, turn on the spray gun to spray the slurry, wet the masterbatch with 3% hypromellose aqueous solution, observe the wetting status of the granules, and turn on the powder supply machine to start powder supply when appropriate . During the granulation process, the main machine speed, spraying speed and powder supply speed can be adjusted in time according ...
Embodiment 2
[0069] Preparation of dabigatran etexilate mesylate capsules
[0070] Dabigatran etexilate mesylate 173g
[0071] Mannitol 45 g
[0072] Silica 8 g
[0073] Crospovidone 25 g
[0074] Sodium stearyl fumarate (additional) 5 g
[0075] Sodium stearyl fumarate (additional) 2 g
[0077] Made into 1000 capsules, wherein the capsule shell is selected from hypromellose capsule shell,
[0078] Specific steps:
[0079] 1. Preprocessing
[0080] Put the silicon dioxide and crospovidone into a blast drying oven at 80°C and dry until the water content is less than 2.5%.
[0081] 2. weighing
[0082] Double-checked and weighed dabigatran etexilate mesylate, mannitol, crospovidone, silicon dioxide and sodium stearyl fumarate, each passed through a 60-mesh sieve for later use.
[0083] 3. Primary mix:
[0084] According to the batch prescription, after checking the dosage by two people, put dabigatran etexilate mesylate, mannitol, crospovidone, silic...
Embodiment 3
[0096] Specification 110mg
[0097] Dabigatran etexilate mesylate 127g
[0098] Mannitol 33 g
[0099] Silica 6 g
[0100] Crospovidone 18 g
[0101] Sodium stearyl fumarate (internal addition) 3.6 g
[0102] Sodium stearyl fumarate (additional) 1.5 g
[0104] Make 1000, wherein capsule shell is selected hypromellose capsule shell, and concrete preparation method is with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com