MMP-8 responsive intelligent periodontal drug controlled release hydrogel material and application thereof
A gel material, MMP-8 technology, applied in the treatment of periodontal disease, MMP-8 responsive intelligent periodontal drug controlled release hydrogel material field, no research that can solve MMP-8 responsiveness has been seen Report and other problems, to achieve the effect of simple drug loading method, good biocompatibility and stable activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
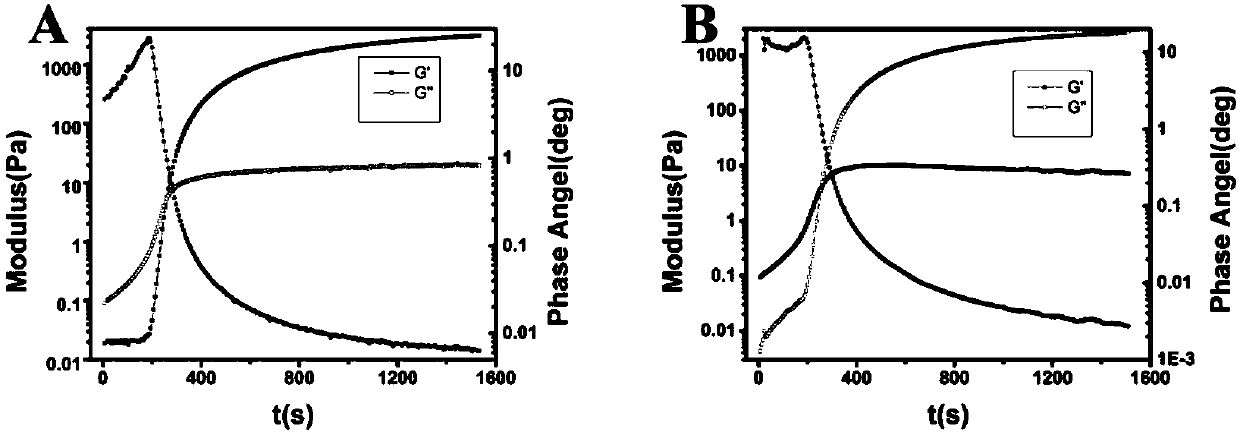
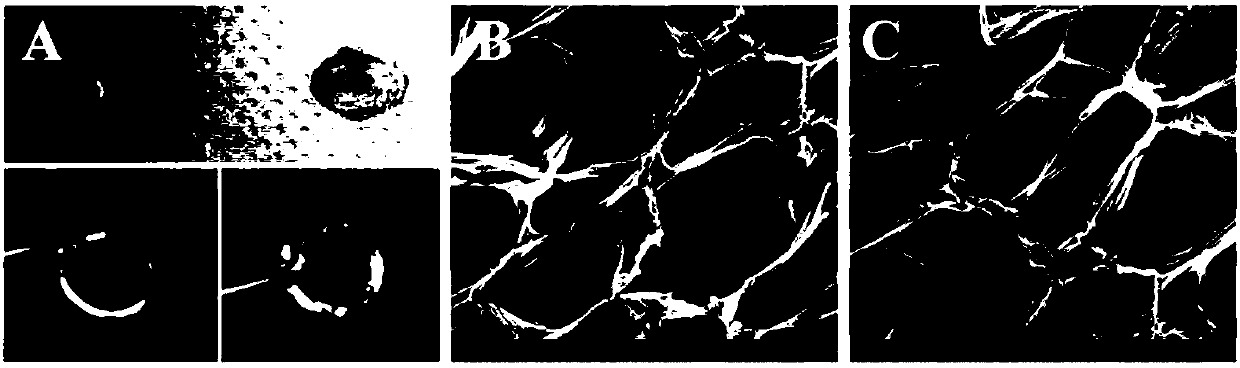
Examples
Embodiment 1
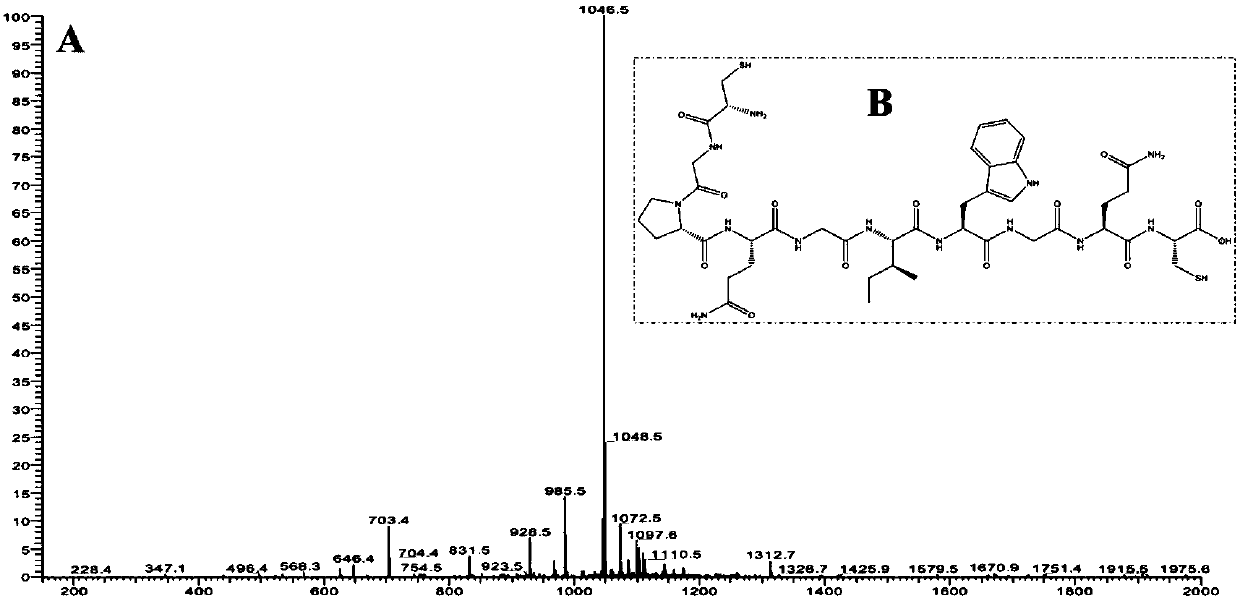
[0054] (1) At room temperature, using 1000 mg of chlorine resin, the sequence synthesized by Fmoc solid-phase synthesis is CGPQGIWQGC (Cys-Gly-Pro-Gln-Gly-Ile-Trp-Gln-Gly-Cys, Cysteine-Glycine-Pro Amino acid-glutamine-glycine-isoleucine-tryptophan-glutamine-glycine-cysteine) MMP-8 sensitive polypeptide (M8SP).
[0055] (2) Using mass spectrometry (ESI) to identify the synthesized polypeptide, the molecular weight is 1048, and it is confirmed that the synthesized polypeptide is the target polypeptide.
[0056] (3) Use high-performance liquid chromatography (HPLC) to detect the purity of the synthesized polypeptide, and purify the polypeptide so that the purity can reach more than 99%.
[0057] (4) Prepare a 0.3 mol / L triethanolamine (TEA) aqueous solution, adjust the pH to 8.0 with a 0.1 mol / L hydrochloric acid solution, and obtain a TEA buffer solution with a pH of 8.0.
[0058] (5) Add 20 mg of the purified M8SP into 1 ml of the above-prepared TEA buffer to obtain an M8SP so...
Embodiment 2
[0064] (1) At room temperature, using 500 mg of chlorine resin, the sequence was synthesized by Fmoc solid-phase synthesis as CGPQGIWQGC (Cys-Gly-Pro-Gln-Gly-Ile-Trp-Gln-Gly-Cys, Cysteine-Glycine-Pro Amino acid-glutamine-glycine-isoleucine-tryptophan-glutamine-glycine-cysteine) MMP-8 sensitive polypeptide (M8SP).
[0065] (2) Identify the synthesized polypeptide by mass spectrometry (ESI), and determine that the synthesized molecular weight is 1048, which is the target polypeptide.
[0066] (3) Use HPLC to detect the purity of the polypeptide, and purify the polypeptide so that the purity can reach more than 99%.
[0067] (4) Prepare a 0.3 mol / L triethanolamine aqueous solution (TEA), adjust the pH to 8.0 with a 0.1 mol / L hydrochloric acid solution, and obtain a TEA buffer solution with a pH of 8.0.
[0068] (5) Add 20 mg of the purified M8SP into 1 ml of the above-prepared TEA buffer to obtain an M8SP solution with a concentration of 20 mg / mL.
[0069] (6) Dissolve 5 mg of ...
Embodiment 3
[0076] (1) At room temperature, using 500 mg of chlorine resin, the sequence was synthesized by Fmoc solid-phase synthesis as CGPQGIWQGC (Cys-Gly-Pro-Gln-Gly-Ile-Trp-Gln-Gly-Cys, Cysteine-Glycine-Pro Amino acid-glutamine-glycine-isoleucine-tryptophan-glutamine-glycine-cysteine) MMP8-sensitive polypeptide (M8SP).
[0077] (2) ESI was used to identify the synthesized polypeptide, and it was determined that the synthesized polypeptide was the target polypeptide with a molecular weight of 1048.
[0078] (3) Use HPLC to detect the purity of the synthesized polypeptide, and purify the polypeptide to make the purity reach more than 99%.
[0079] (4) A 0.3 mol / L triethanolamine water (TEA) solution was prepared, and the pH was adjusted to 8.0 with a 0.1 mol / L hydrochloric acid solution to obtain a TEA buffer solution with a pH of 8.0.
[0080] (5) Add 20 mg of the purified M8SP into 1 ml of the above-prepared TEA buffer to obtain an M8SP solution with a concentration of 20 mg / mL.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com