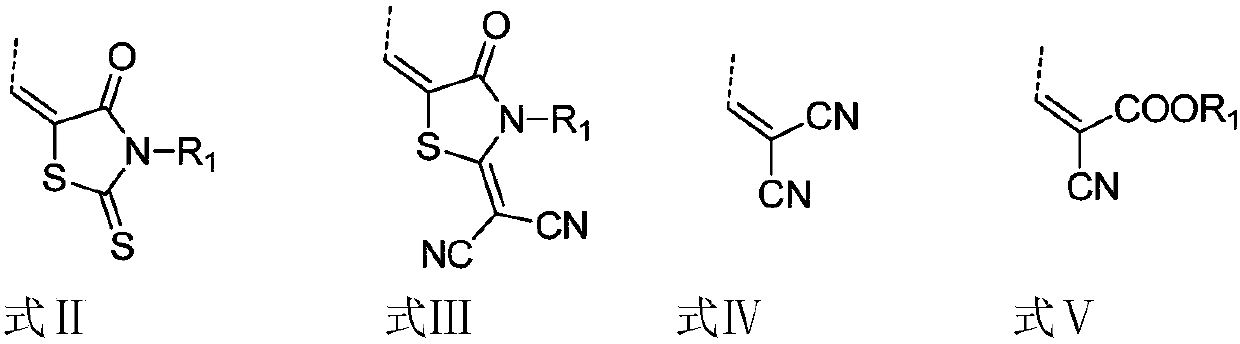
Preparation method for broad-band gap star-shaped non-fullerene micromolecule receptor and application of preparation method
A small molecule acceptor, non-fullerene technology, used in semiconductor/solid-state device manufacturing, electric solid-state devices, semiconductor devices, etc., can solve the problems of large phase separation, affecting the energy conversion efficiency of organic solar cells, and poor morphology. , to achieve the effect of high energy conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] An organic small molecule receptor with a chemical structure of 2,7,12-TrBRCN, the synthesis route of which is as follows:
[0027]
[0028] Compound 1 can be reported according to the literature (J.Am.Chem.Soc., 2016, 138, 2528-2531; J.Am.Chem. Soc., 2015, 137, 3901-3909; Nature Communications, 2016, 7, 12469) step synthesis. Compound 5 can be synthesized according to the steps reported in literature (J.Am.Chem.Soc.2015,137,898-904).
[0029] Synthesis of compound 2: Dissolve compound 1 (0.30g, 0.39mmol), diboronic acid pinacolone ester (0.69g, 2.70mmol) potassium acetate (0.30g, 3.05mmol) in dry DMF (30mL), remove oxygen , under nitrogen protection, adding PdCl 2 (dppf) 2 (0.029g, 0.04mmol), reflux for 48h. After the reaction, DMF was removed under reduced pressure, extracted with dichloromethane, and dried over anhydrous magnesium sulfate. After filtration, the crude product was separated and purified by column chromatography (silica gel; eluent: petroleum et...
Embodiment 2
[0037] A small organic molecule receptor with a chemical structure of 3,8,13-TrBRCN, the synthesis route of which is as follows:
[0038]
[0039] Compound 4 can be synthesized according to the steps reported in literature (Chem. Eur. J. 2015, 21, 13052-13057).
[0040] Synthesis of Compound 5: Compound 4 (3.28g, 15.6mmol), p-toluenesulfonic acid monohydrate (12.7g, 66.6mmol), propionic acid (3.69g, 49.7mmol)) were dissolved in 15mL o-dichlorobenzene . The reaction solution was heated to 105 °C and stirred for 16 h. After cooling to room temperature, the reaction solution was poured into methanol, neutralized with sodium hydroxide, and the solid was collected by filtration. The obtained solid was washed with methanol, ethanol, and recrystallized with tetrachloroethane to obtain product 5 as a pale yellow solid (2.54 g, yield: 84%). Synthesis of Compound 6: Compound 5 (1.1 g, 1.91 mmol) and potassium tert-butoxide (4.28 g, 38.2 mmol) were added to dry tetrahydrofuran (80 ...
Embodiment 3
[0049] Absorption Spectra Measurement of Small Organic Molecule Receptors 2,7,12-TrBRCN and 3,8,13-TrBRCN
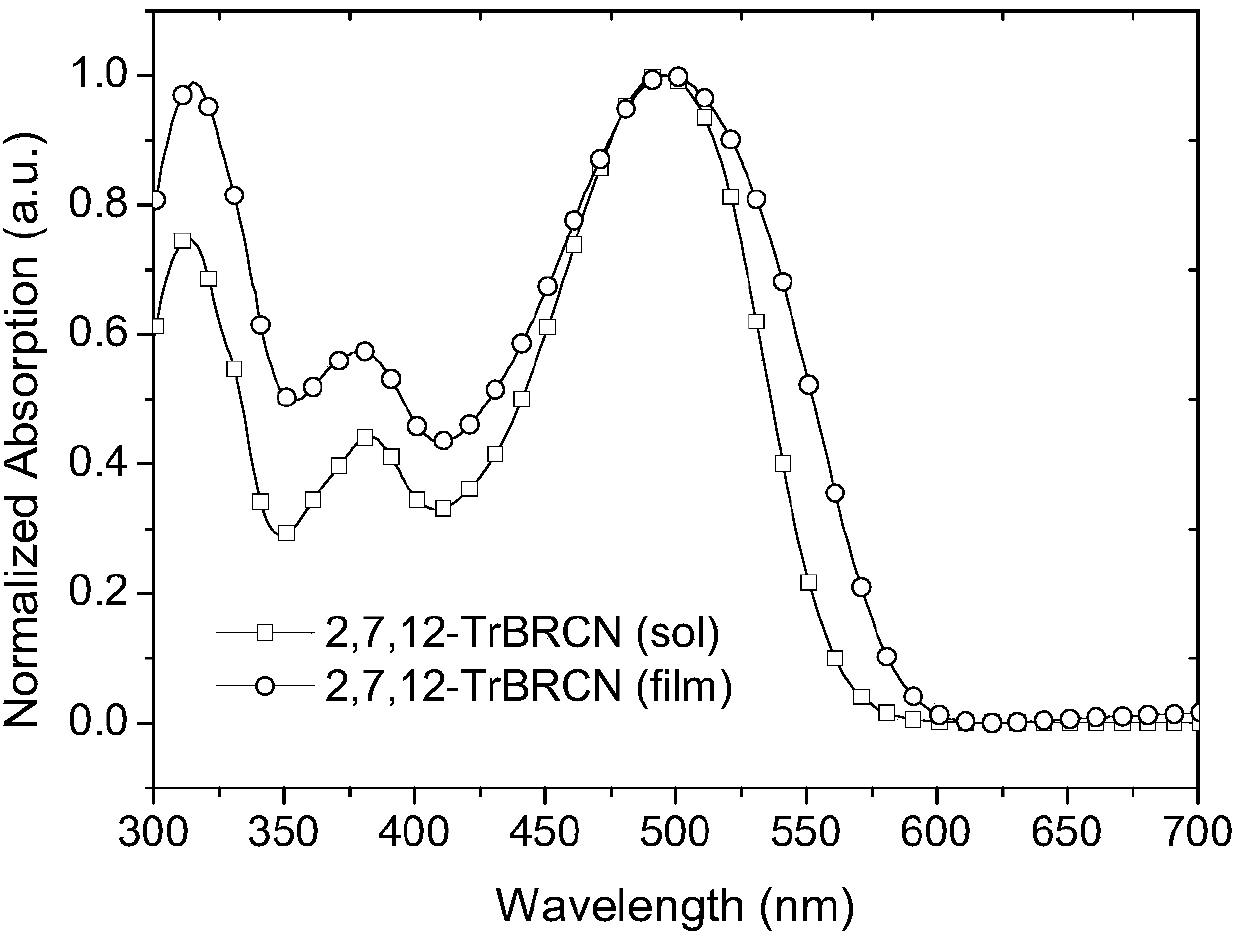
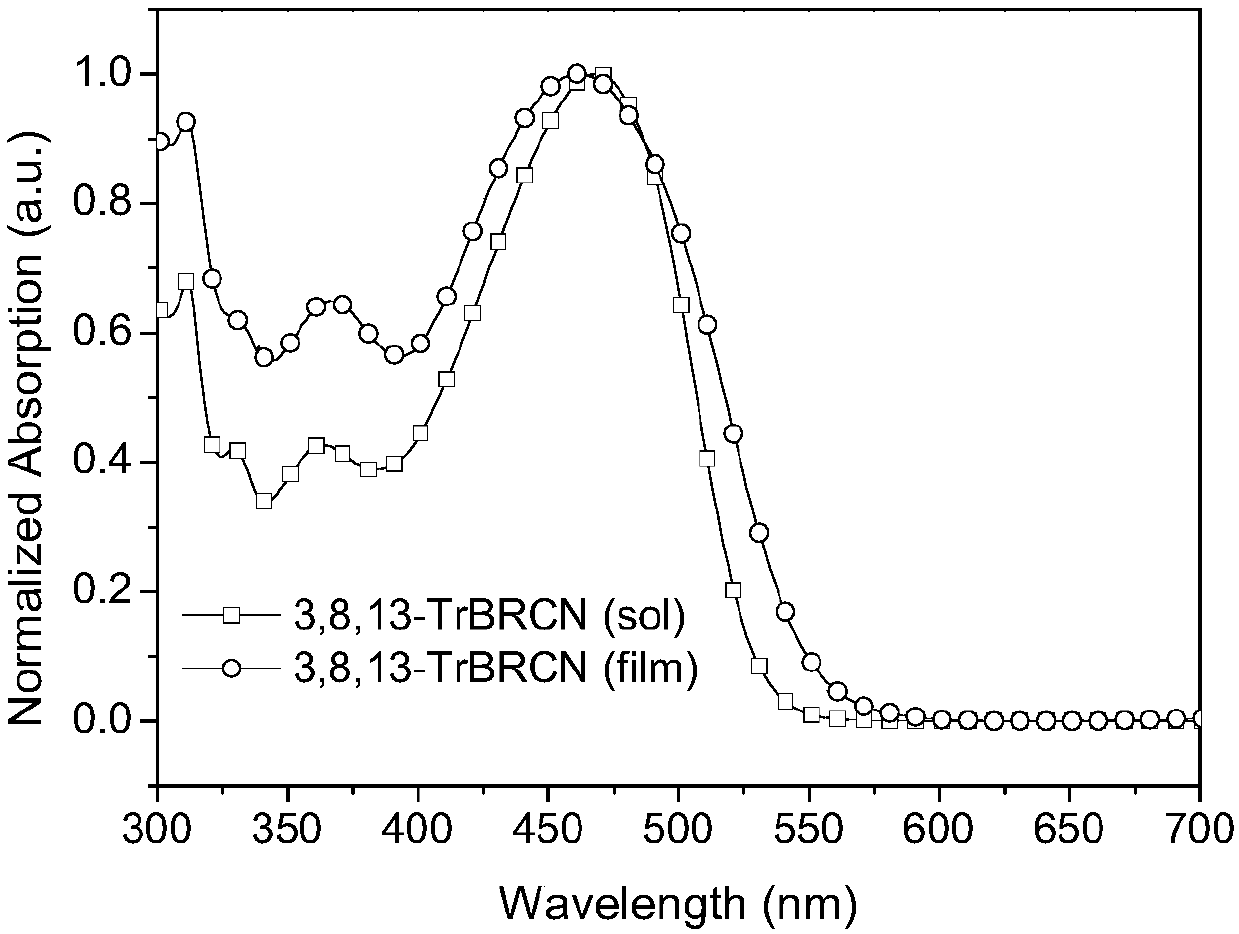
[0050] figure 1 and figure 2 UV-Vis absorption spectra of small molecule acceptors 2,7,12-TrBRCN and 3,8,13-TrBRCN in chloroform solution and on quartz plate, respectively.
[0051] Depend on figure 1 It can be seen that the maximum absorption value of 2,7,12-TrBRCN film is about 475nm, the peak value is about 575nm, and its optical bandgap is 2.16eV (the optical bandgap is based on the formula E g =1240 / λ onset calculation, where E g is the optical bandgap, λ onset is the peak value of film absorption).
[0052] Depend on figure 2 It can be seen that the maximum absorption value of 3,8,13-TrBRCN film is about 500nm, the peak value is about 600nm, and its optical bandgap is 2.07eV (the optical bandgap can be calculated according to the formula E g =1240 / λ onset calculation, where E g is the optical bandgap, λ onset is the maximum absorption sideband value ab...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Optical bandgap | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Short circuit current | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com