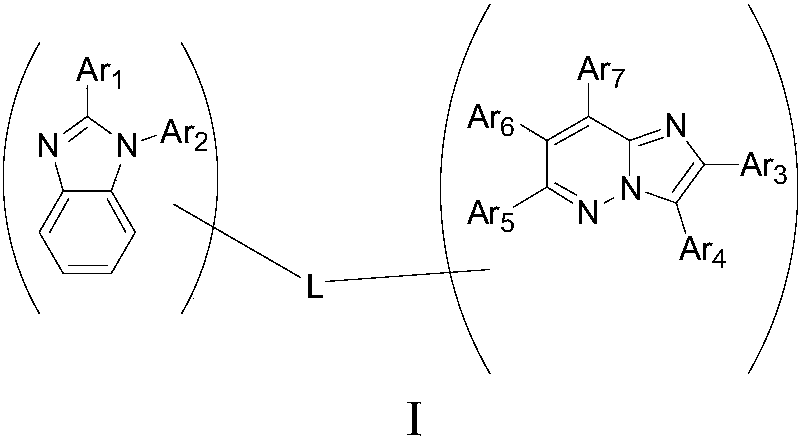
Organic electroluminescence compound based on imidazole and pyridazine as well as application and organic electroluminescence device thereof
An imidazopyridazine, electroluminescence technology, applied in the direction of organic chemistry, electro-solid devices, electrical components, etc., can solve the problems affecting the life and efficiency of devices, low electron mobility, low electron mobility, etc., and achieve good thermal stability. The effect of high luminous efficiency, high luminous efficiency and high luminous purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
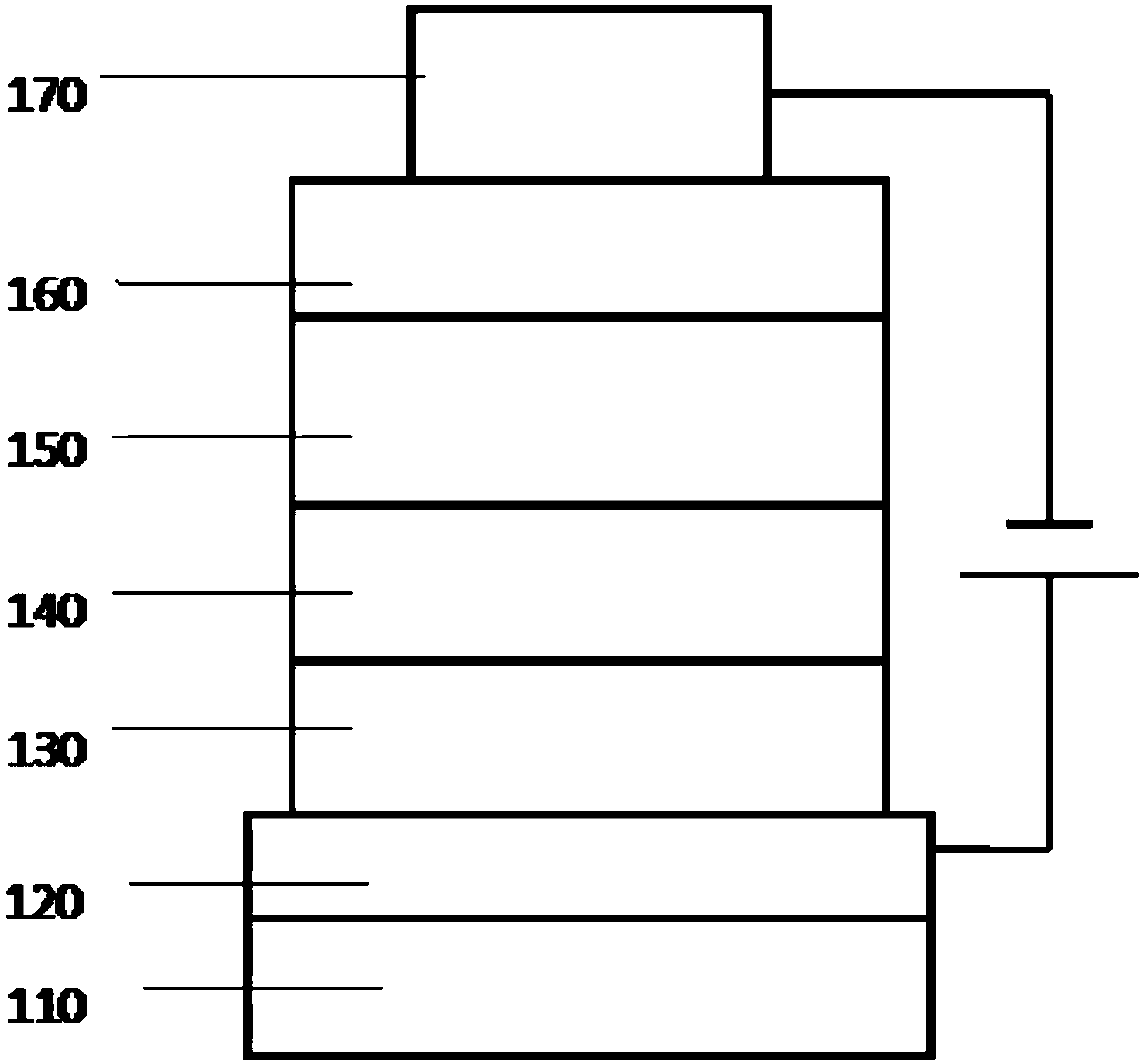
Image
Examples
Embodiment 1
[0044] Synthetic route of compound 1
[0045]
[0046] The synthetic method of intermediate 1-1
[0047] In the flask, add 2-bromoacetophenone (10g, 50mmol), 3-amino-6-chloropyridazine (6.5g, 50mmol), ethanol (80mL), heat and reflux for 6 hours, cool, and filter to obtain the product 7.7 g, 67% yield.
[0048] The synthetic method of compound 1
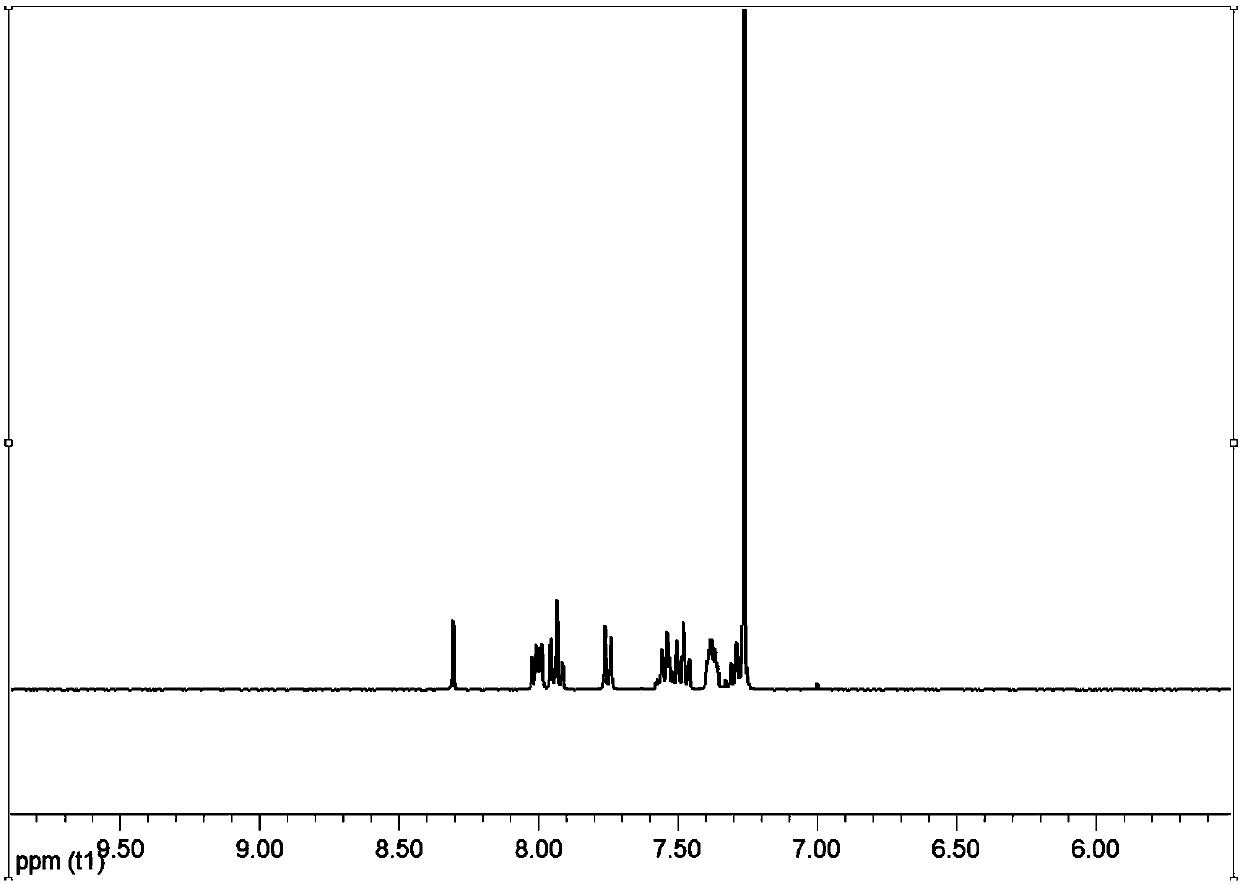
[0049] In a flask, add intermediate 1-1 (1 g, 4.4 mmol), B-[4-(1-phenyl-1H-benzimidazol-2-yl)phenyl]-boronic acid (1.4 g, 4.4 mmol) , tetrakistriphenylphosphine palladium (0.1g), potassium carbonate (1.4g, 10mmol), tetrahydrofuran (15mL), water (10mL), heated to reflux for 10 hours under nitrogen protection, cooled, extracted with dichloromethane, dried, Concentrate, obtain product through column chromatography purification and be 1.7g, productive rate is 83%, and its NMR spectrum is as follows: figure 1 shown.
Embodiment 2
[0051] Synthetic route of compound 7
[0052]
[0053] The synthetic method of intermediate 7-1
[0054] In the flask, add 2-bromoacetophenone (10g, 50mmol), 3-amino-4-bromo-6-chloropyridazine (10.4g, 50mmol), add ethanol (100mL), heat and reflux for 5 hours, cool , and filtered to obtain 14 g of the product with a yield of 92%.
[0055] The synthetic method of intermediate 7-2
[0056] In a flask, add Intermediate 7-1 (8g, 26mmol), 4-biphenylboronic acid (5.2g, 26mmol), potassium carbonate (7.2g, 52mmol), tetrahydrofuran (100mL), water (50mL), tetrakistriphenyl Palladium phosphine (0.3g), heated to reflux for 6 hours under the protection of nitrogen, cooled, filtered, and the filter cake was recrystallized with ethanol and tetrahydrofuran to obtain 5.7g of the product with a yield of 58%. The synthetic method of compound 7
[0057] The synthesis method was the same as that of compound 1, except that intermediate 1-1 was replaced by intermediate 7-2, and the yield was 7...
Embodiment 3
[0059] Synthetic route of compound 40
[0060]
[0061] The synthetic method of intermediate 40-1
[0062] The synthesis method is the same as that of compound 1, the raw materials used are intermediate 1-1 and 3-chlorophenylboronic acid, and the yield is 54%.
[0063] The synthetic method of compound 40
[0064] In a flask, add intermediate 40-1 (1.5g, 4.9mmol), B-[3-(1-phenyl-1H-benzimidazol-2-yl)phenyl]-boronic acid (1.5g, 4.9mmol ), palladium acetate (0.1g), X-phos (0.2g), potassium carbonate (1.4g, 10mmol), dioxane (15mL), water (10mL), heated to reflux under nitrogen protection for 10 hours, cooled, It was extracted with dichloromethane, dried, concentrated, and purified by column chromatography to obtain 1.4 g of the product with a yield of 54%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com