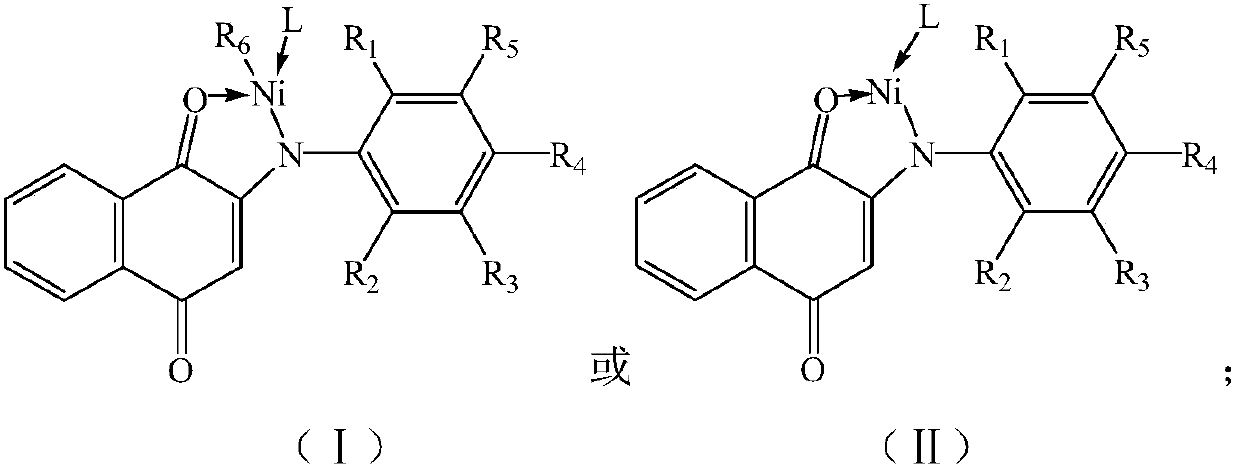
Heterogeneous polymerization reaction catalyst and application of heterogeneous polymerization reaction catalyst to preparing homopolymer and copolymer
A polymerization reaction and catalyst technology, which is applied in the application field of heterogeneous polymerization reaction catalyst, preparation of homopolymers and copolymers, can solve the problems of poor polar functional group tolerance and low catalytic activity, and achieves good tolerance. , the effect of high catalytic activity and low degree of branching
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] The preparation method of catalyst B, the steps are as follows:
[0070] (1) Dissolve 2-hydroxy-1,4-naphthoquinone and substituted aniline in n-heptane, add trifluoroacetic acid to react to obtain anilinonaphthoquinone ligands, the reaction temperature is 100°C, the time is 15h, the reaction Initially, the molar ratio of 2-hydroxy-1,4-naphthoquinone to substituted aniline was 1:1.15, and the molar ratio of trifluoroacetic acid to 2-hydroxy-1,4-naphthoquinone was 0.33:1,2-hydroxy- The concentration of 1,4-naphthoquinone in n-heptane is 0.125mol / L, and the structural formula of substituted aniline is as follows:
[0071]
[0072] In the formula, R 1 is methyl, R 2 is ethyl, R 3 is methyl, R 4 is ethyl, R 5 It is a methyl group, and after the reaction, the anilinaquinone ligands are purified by toluene recrystallization;
[0073] (2) Dissolving the anilino-naphthoquinone ligands in toluene, adding sodium hydride to react to obtain a ligand salt compound, the tempe...
Embodiment 2
[0079] The preparation method of catalyst B, the steps are as follows:
[0080] (1) Dissolve 2-hydroxy-1,4-naphthoquinone and substituted aniline in toluene, add trifluoroacetic acid to react to obtain aniline naphthoquinone ligands, the reaction temperature is 110°C, the time is 6h, when the reaction starts , the molar ratio of 2-hydroxy-1,4-naphthoquinone to substituted aniline is 1:1, the molar ratio of trifluoroacetic acid to 2-hydroxy-1,4-naphthoquinone is 0.32:1, 2-hydroxy-1, The concentration of 4-naphthoquinone in toluene is 0.1mol / L, and the structural formula of substituted aniline is as shown in embodiment 1, and in the formula, R 1 is ethyl, R 2 is methyl, R 3 is ethyl, R 4 is methyl, R 5 After the reaction, the anilinaquinone ligands are purified by n-hexane extraction;
[0081] (2) Dissolving the anilinaquinone ligands in tetrahydrofuran, adding potassium hydride to react to obtain a ligand salt compound, the reaction temperature is 40°C, and the time is 1h....
Embodiment 3
[0085] The preparation method of catalyst B, the steps are as follows:
[0086] (1) Dissolve 2-hydroxy-1,4-naphthoquinone and substituted aniline in chlorobenzene, add trifluoroacetic acid to react to obtain anilino-naphthoquinone ligands, the reaction temperature is 130°C, the time is 6h, and the reaction starts , the molar ratio of 2-hydroxy-1,4-naphthoquinone to substituted aniline is 1:1.02, and the molar ratio of trifluoroacetic acid to 2-hydroxy-1,4-naphthoquinone is 0.32:1,2-hydroxy-1 , the concentration of 4-naphthoquinone in chlorobenzene is 0.11mol / L, and the structural formula of substituted aniline is as shown in embodiment 1, and in the formula, R 1 is isopropyl, R 2 is tert-butyl, R 3 is tert-butyl, R 4 is isopropyl, R 5It is isopropyl, and after the reaction, the p-aniline ligands are purified by recrystallization from tetrahydrofuran;
[0087] (2) Aniline naphthoquinone ligands are dissolved in dichloromethane, and n-butyllithium is added to react to obtai...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com