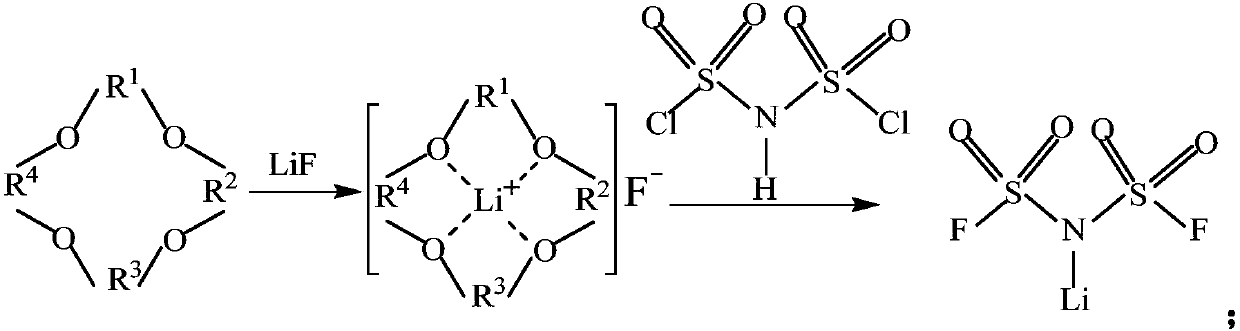
Method for preparing imidodisulfuryl fluoride lithium with villiaumite as fluorinating agent
A technology of lithium bisfluorosulfonimide and bischlorosulfonimide is applied in the field of preparation of lithium bisfluorosulfonimide, which can solve the problems of high reaction risk, complicated operation, high toxicity and the like, and achieves reaction conditions Mild, simple follow-up treatment, minimal impact on preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of Dichlorosulfonimide (ie HClSI)
[0033] Will NH 2 SO 3 H was added to an anhydrous and oxygen-free reaction flask, and SOCl was added under stirring 2 , when the temperature of the reaction system rises to 80°C, the ClSO 3 H was slowly added dropwise into the reaction bottle, and after the drop, the reaction temperature was raised to 130°C, and the reaction continued for 24 hours until no tail gas was generated. During the process, the color of the reaction solution gradually turned brownish red, and the amount of the reaction solution was very Significant reduction of HN(SO 2 Cl) 2 initial product.
[0034] The obtained product is subjected to vacuum distillation at 82-85° C., and the obtained product is a transparent solution. The properties of the product were detected by NMR and LG-MS. The yield of this reaction was integrated to be about 95%.
[0035] 1 H-NMR (CDCl 3 ): δ8.76
[0036] 19 F-NMR (CDCl 3 ): δ58.12
[0037] Reaction formula: ...
Embodiment 2
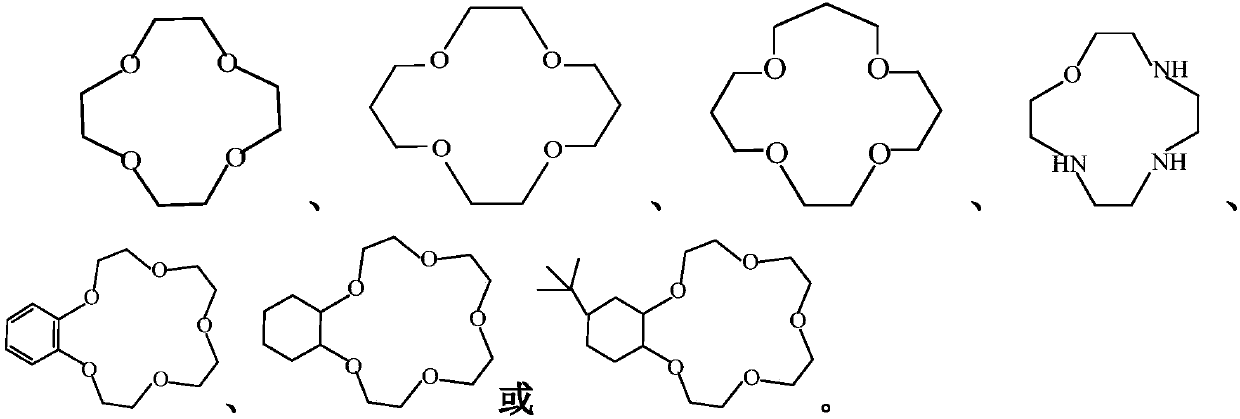
[0040] Under nitrogen and deoxygenation conditions, add an appropriate amount of LiF (fluorinating agent) and complexing agent 12-crown-4 (1 to 5% of the amount of LiF) into the reaction bottle, and add an appropriate amount of acetonitrile or carbonic acid after water removal treatment. Diethyl ester dissolves LiF and 12-crown-4. Add 20-100 g of HClSI to the reactor under stirring, continue the reaction at a low temperature of -10-0° C. for 10 h, then end the reaction, and collect the filtrate. The solvent was distilled off under reduced pressure to obtain a light yellow viscous concentrated liquid, which was determined by 19 F-NMR, LG-MS and other spectra. The yield of this reaction was integrated to be about 88%.
[0041] 19 F-NMR (CDCl 3 ): δ51.56
[0042] Reaction formula:
[0043]
Embodiment 3
[0045]Under the condition of nitrogen and oxygen removal, add appropriate amount of LiF (fluorinating agent) and complexing agent 14-crown-4 (1 to 5% of the amount of LiF) into the reaction bottle, and add appropriate amount of acetonitrile or carbonic acid after water removal treatment. Diethyl ester dissolves LiF and 14-crown-4. Add 20-100g of HClSI to the reactor under stirring, continue the reaction at a low temperature of -10-0°C for 10 hours, then end the reaction, and collect the filtrate. The solvent was distilled off under reduced pressure to obtain a light yellow viscous concentrated solution, which was determined for 19 F-NMR, LG-MS and other spectra. The yield of this reaction was integrated to be about 80%.
[0046] 19 F-NMR (CDCl 3 ): δ51.53
[0047] Reaction formula:
[0048]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com