Preparation method of pyrazole derivative
A technology of pyrazole derivatives and derivatives, applied in hydrazone preparation, organic chemistry, etc., can solve the problems of low yield, harsh reaction conditions, inconvenient operation, etc., and achieve ordinary reaction conditions, high reaction yield and high product quality high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
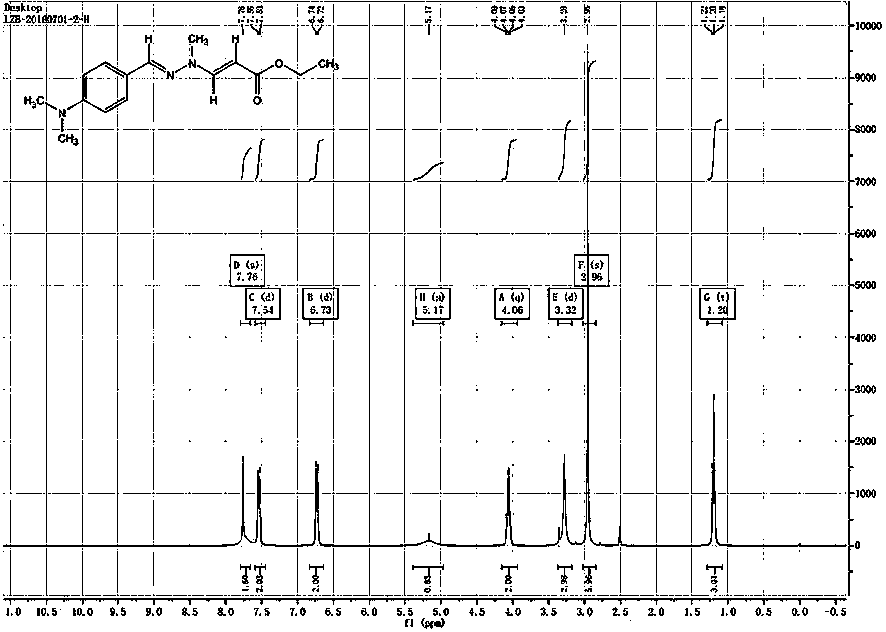
[0068] One, the preparation method of the intermediate compound shown in formula IV of the present invention, concrete steps are as follows:
[0069] First, the p-substituted phenyl derivative shown in formula I reacts with the hydrazine compound shown in formula II to generate the hydrazone compound shown in formula III,
[0070] ,
[0071] in,
[0072] R 1 for OR A or NR B R C , where R A C1~C8 alkyl, R B C1~C8 alkyl, R C is C1~C8 alkyl; R 6 is a hydrogen atom or a C1-C8 alkyl group, R 7 It is a C1-C8 alkyl group.
[0073] R 6 When it is a hydrogen atom, the para-substituted phenyl derivative shown in formula I is a para-substituted benzaldehyde derivative; R 6 When it is an alkyl group, the para-substituted phenyl derivative shown in formula I is a para-substituted phenyl alkyl ketone derivative.
[0074] Then by one of the following three methods (preferably the third method), the intermediate compound shown in formula IV is synthesized.
[0075] ,
[0...
Embodiment 1
[0097] The synthesized pyrazole derivative was 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid (DFPA).
[0098] The preparation method of 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid of the present embodiment comprises the following steps:
[0099] Step 1. Synthesis of fluorine-containing intermediate compound (VI).
[0100] In the three-necked round bottom flask, add the intermediate compound (Ⅳ, R 1 for -N(CH 3 ) 2 , R 6 is a hydrogen atom, R 2 for-OC 2 h 5 ) 275g, (1.0 mol) and dichloromethane 1375ml, under the protection of nitrogen, the temperature was lowered to 5°C, and 108g, (1.1 mol) of difluoroacetyl fluoride gas was introduced. During the ventilation process, the temperature of the system was kept below 30°C, and three After passing through 110 grams of 1.08 (mol) ethylamine, it was incubated and stirred for 2 hours to obtain a methylene chloride solution of the fluorine-containing intermediate compound (VI). The gas phase purity was gr...
Embodiment 2
[0109] The synthesized pyrazole derivative was 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid (DFPA).
[0110] The preparation method of 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid of the present embodiment comprises the following steps:
[0111] Step 1. Synthesis of fluorine-containing intermediate compound (VI).
[0112] In the three-necked round bottom flask, add the intermediate compound (Ⅳ, R 1 for -N(CH 3 ) 2 , R 6 is a hydrogen atom, R 2 for -CH 3 ) 245g, (1.0 mol) and dichloromethane 1375ml, under the protection of nitrogen, the temperature was lowered to 5°C, and 108g, (1.1 mol) of difluoroacetyl fluoride gas was introduced. During the ventilation process, the temperature of the system was kept below 20°C, and three Ethylamine 110 grams, heat preservation and stirring for 2 hours after passing through to obtain a dichloromethane solution of the fluorine-containing intermediate compound (VI), the gas phase purity is greater than 95%, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com