Redox-induced pH-responsive methacrylate fluorine-containing monomer, synthesis method and application
A type of methacrylic acid and methacrylate technology, which is applied in the field of redox-induced pH-responsive methacrylate fluorine-containing monomers, can solve the problem of shortening, increase of fluorine signal peak width, and decrease of imaging signal-to-noise ratio and other problems, to achieve the effect of stable quality, simple synthesis method and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The synthesis of embodiment 1 monomer M1
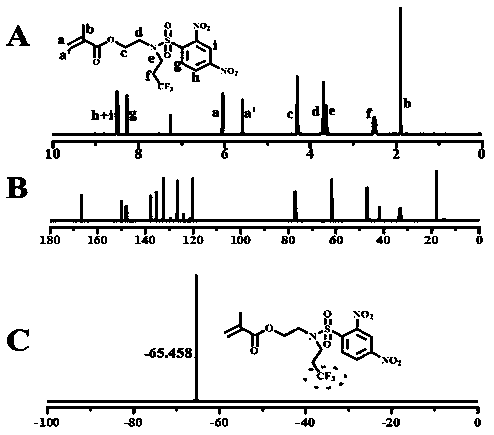
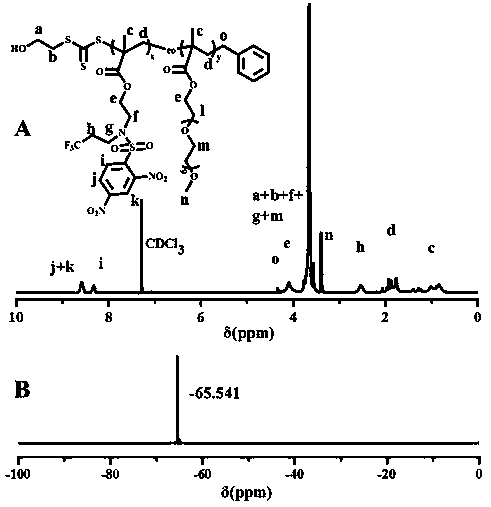
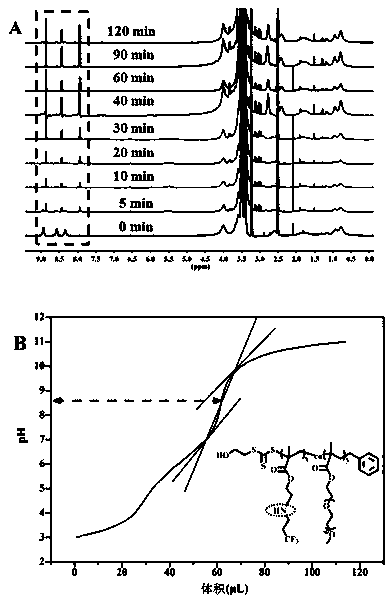
[0026] The chemical structure of monomer M1 is shown below. The specific synthesis steps are as follows: First, add 2-aminoethyl methacrylate hydrochloride (1.65 g, 10 mmol) and triethylamine (2.02 g, 20 mmol) in a 100 mL round bottom flask, add 50 mL of Tetrahydrofuran was dispersed and dissolved in water, and a tetrahydrofuran solution of 2,4-dinitrobenzenesulfonyl chloride (2.66 g, 10 mmol) was added dropwise under ice-bath conditions. After the addition was complete, the solution was returned to room temperature for 10 h. The reaction solution was distilled off the solvent under reduced pressure, and then separated by column chromatography, using ethyl acetate / petroleum ether mixture as the eluent, collecting the eluate containing the target compound, and after the solvent was evaporated, the intermediate product 2- ((2,4-Dinitrophenyl) sulfonamide) ethyl methacrylate, yield 85%. Next, add 2-((2,4-dinitrophenyl)sulfonamid...
Embodiment 2
[0030] Adjust the molar ratio of 3,3,3-trifluoropropan-1-ol to 2-((2,4-dinitrophenyl)sulfonamide)ethyl methacrylate to 2.5:1, and the others are the same as in the examples 1. The obtained product 2-((2,4-dinitro-N-(3,3,3-trifluoropropyl)phenyl)sulfonamide ethyl methacrylate has a yield of 90%.
Embodiment 3
[0032] The reaction temperature was adjusted to 40°C, and the others were the same as in Example 1. The resulting product 2-((2,4-dinitro-N-(3,3,3-trifluoropropyl)phenyl)sulfonamide methacrylic acid Ethyl ester, yield 91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com