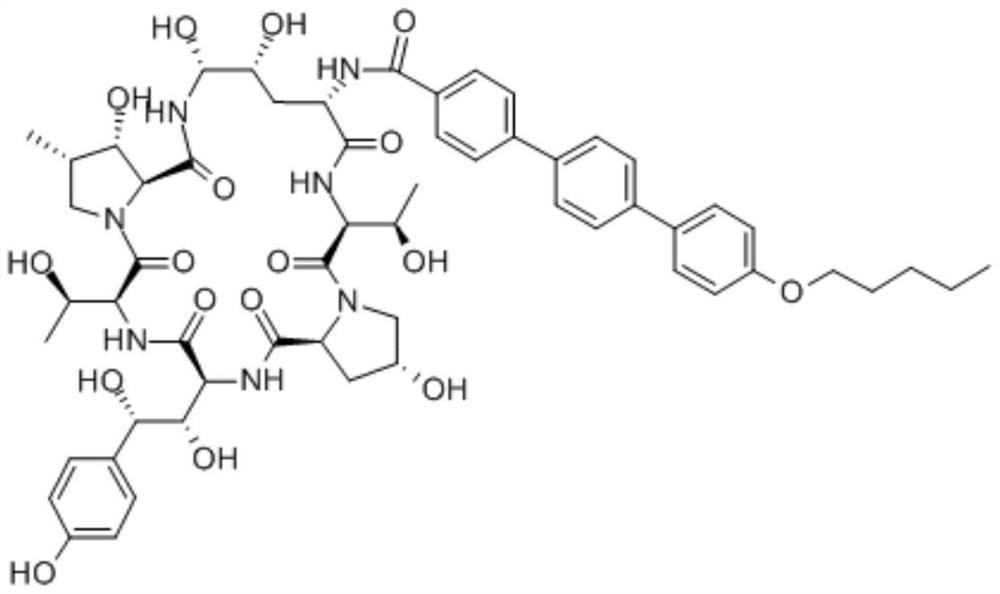
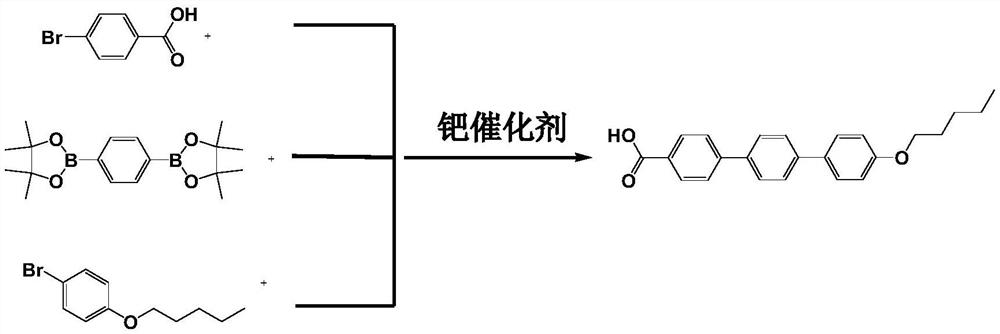
One-step method for the preparation of anidulungin intermediate p-amyloxy terbenzoic acid
A technology of p-pentoxy terbenzoic acid and anidulungin, which is applied in the field of preparation of p-amyloxy terbenzoic acid, an intermediate of anidylfungin, in one-step synthesis, and can solve the problems of high production risk, low production cost, High production cost and other problems, to achieve the effect of low production cost, less three wastes, and short preparation route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 30L of N,N-dimethylformamide to the prepared potassium carbonate solution (7600g potassium carbonate + 30L water) at room temperature, and then add 2000g of 4-bromobenzoic acid and 3650g of 1,4-benzenedi Pinacol borate. The system was replaced with nitrogen for 3 times, and 100 g of tetrakis(triphenylphosphine)palladium was added as a catalyst. Under the protection of nitrogen, the temperature was slowly raised to 73°C and stirred for 7 hours. Keeping the temperature constant, 2450 g of 4-n-pentyloxybromobenzene and 5 L of dimethylformamide solution were slowly added dropwise. After 4 hours of dropping, the reaction was continued for 8 hours. After the completion of the reaction, cool to 8°C, add 0.5N dilute hydrochloric acid aqueous solution to the system dropwise under stirring until the system pH=5, and stir for 2 hours after the addition. Suction filtration, the filter cake was rinsed twice with water and once with ethanol to obtain the target product anifungin...
Embodiment 2
[0034] Add 40L of N,N-dimethylformamide to the prepared sodium carbonate solution (5000g sodium carbonate + 25L water) at room temperature, and then add 1600g of 4-bromobenzoic acid and 3000g of 1,4-benzenedi Pinacol borate. The system was replaced with nitrogen for 3 times, and 95 g of [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride was added as a catalyst. Under the protection of nitrogen, the temperature was slowly raised to 76°C and stirred for 7 hours. Keeping the temperature constant, 1950 g of 4-n-pentyloxybromobenzene and 4.7 L of dimethylformamide solution were slowly added dropwise. After 3.5 hours of completion of the dropwise addition, the reaction was continued for 8 hours. After the reaction was completed, cool to 8° C. with a water bath, add 0.5 M dilute hydrochloric acid aqueous solution to the system dropwise with stirring until the system pH=4, and stir for 2 h after the addition. Suction filtration, the filter cake was rinsed twice with water and...
Embodiment 3
[0036] Add 20L of N,N-dimethylformamide to the prepared cesium carbonate solution (8600g cesium carbonate + 20L water) at room temperature, and then add 1000g of 4-bromobenzoic acid and 1800g of 1,4-benzenedi Pinacol borate. The system was replaced with nitrogen for 3 times, and 40 g of palladium acetate was added as a catalyst. Under the protection of nitrogen, the temperature was slowly raised to 75°C and stirred for 7 hours. Keeping the temperature constant, 1220 g of 4-n-pentyloxybromobenzene and 3 L of dimethylformamide solution were slowly added dropwise. After 2 hours of dropwise addition, the reaction was continued for 8 hours. After the reaction was completed, cool to 6° C. with a water bath, add 0.5 M dilute hydrochloric acid aqueous solution to the system dropwise with stirring until the system pH=5, and stir for 2 h after the addition. Suction filtration, the filter cake was rinsed twice with water and once with ethanol to obtain the target product anifungin inter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com