Solid composition containing rivaroxaban and preparation method thereof
A solid composition, the technology of rivaroxaban is applied in the directions of medical preparations containing active ingredients, medical preparations without active ingredients, drug combinations, etc. Rivaroxaban has problems such as large particle size, and achieves the effect of small total impurities, ensuring safety and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
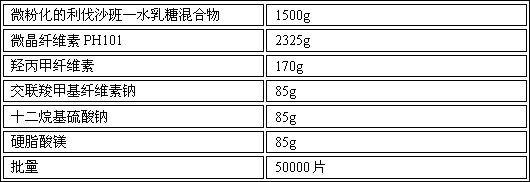
[0066] Embodiment 1: prepare with 50,000 prescription quantities
[0067] Product Prescription:
[0068]
[0069] Preparation Process:
[0070] 1. Crushing of raw materials: mix rivaroxaban and lactose monohydrate at a ratio of 1:2, pass through a 60-mesh sieve twice, and grind to a particle size (D 90 ) is less than 10 μm, to obtain micronized rivaroxaban monohydrate lactose mixture;
[0071] 2. Dissolve the prescribed amount of hypromellose and sodium lauryl sulfate in purified water to obtain solution I;
[0072] 3. Mixing: Weigh the prescription amount of micronized rivaroxaban lactose monohydrate mixture, microcrystalline cellulose PH101, and croscarmellose sodium and mix them in a mixer, stir and mix at a low speed for 5 minutes, until dry mixed powder;
[0073] 4. Granulation: Add solution Ⅰ into the dry mixed powder under low-speed stirring to make soft material, and granulate the soft material with a oscillating granulator (16-mesh nylon screen) to obtain wet g...
Embodiment 2
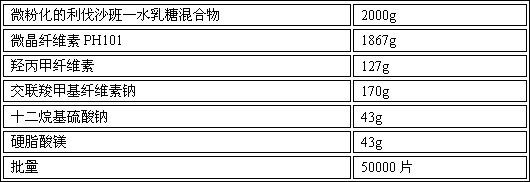
[0085] Embodiment 2: prepare with 50,000 prescription quantities
[0086] Product Prescription:
[0087]
[0088] Preparation Process:
[0089] 1. Grinding of raw materials: mix rivaroxaban and lactose monohydrate at a ratio of 1:3, pass through a 60-mesh sieve twice, and grind to a particle size (D 90) is less than 10 μm, to obtain a micronized rivaroxaban monohydrate lactose mixture;
[0090] 2. Dissolve the prescribed amount of hypromellose and sodium lauryl sulfate in purified water to obtain solution I;
[0091] 3. Mixing: Weigh the prescribed amount of micronized rivaroxaban lactose monohydrate mixture, microcrystalline cellulose PH101, and croscarmellose sodium, mix them in a mixer, stir and mix at a low speed for 8 minutes, and dry mixed powder;
[0092] 4. Granulation: Add solution Ⅰ into the dry mixed powder under low-speed stirring to make soft material, and granulate the soft material with a oscillating granulator (16-mesh nylon screen) to obtain wet granule...
Embodiment 3
[0101] Embodiment 3: Prepare with 50,000 prescription quantities
[0102] Product Prescription:
[0103] Micronized rivaroxaban monohydrate lactose mixture
2500g
Microcrystalline Cellulose PH101
1367g
127g
170g
43g
43g
batch
50000 pieces
[0104] Preparation Process:
[0105] 1. Crushing of raw materials: mix rivaroxaban and lactose monohydrate at a ratio of 1:5, pass through a 60-mesh sieve twice, and grind to a particle size (D 90 ) is less than 10 μm, to obtain a micronized rivaroxaban monohydrate lactose mixture;
[0106] 2. Dissolve the prescribed amount of hypromellose and sodium lauryl sulfate in purified water to obtain solution I;
[0107] 3. Mixing: Weigh the prescription amount of micronized rivaroxaban lactose monohydrate mixture, microcrystalline cellulose PH101, and croscarmellose sodium and mix them in a mix...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com