Preparation method of human-derived copper-zinc superoxide dismutase
A superoxide and dismutase technology, applied in biochemical equipment and methods, oxidoreductase, enzymes, etc., can solve the problems of low target protein expression, complicated purification process, affecting target protein expression, etc., to avoid Safety risks, improved expression, and beneficial effects on purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The experimental methods used in the examples of the present invention are conventional methods unless otherwise specified.
[0044] The materials and reagents used in the examples of the present invention can be obtained from commercial sources unless otherwise specified.
[0045] The nucleotide fragment comprising hSOD1 gene coding sequence used in the present invention can be obtained by PCR amplification, artificial synthesis and other methods. For artificially synthesizing the target gene, those skilled in the art know techniques such as analyzing codon bias by software such as Emboss and designing the target gene sequence. Different organisms have a certain preference for codons encoding the same amino acid. Through the analysis of codon usage preference, the design of these optimal codons can improve the expression of the target gene. In the present invention, the nucleotide fragment comprising the coding sequence of the hSOD1 gene is preferably artificially syn...
Embodiment 2
[0063] Embodiment 2: Construction of recombinant escherichia coli
[0064] 1) Preparation of Escherichia coli Competent Cells
[0065] with CaCl 2 Preparation of Escherichia coli competent cells by chemical method, the steps include: taking 1ml of activated Escherichia coli bacteria liquid and inoculating it into 100ml LB medium; shaking culture at 37°C until OD 600 = 0.35-0.5 hours; collect the bacteria by centrifugation at 4000g at 4°C; add 40ml of pre-cooled 0.1MCaCl 2 Resuspend the cells and keep on ice for 10 minutes; collect the cells by centrifugation at 4000g at 4°C; add 2ml of pre-cooled 0.1M CaCl 2 Resuspend the cells; add glycerol with a final concentration of 15% to 20%; aliquot into 50 μL per tube; store at 4°C for 24 hours and transfer to a -80°C refrigerator for freezing.
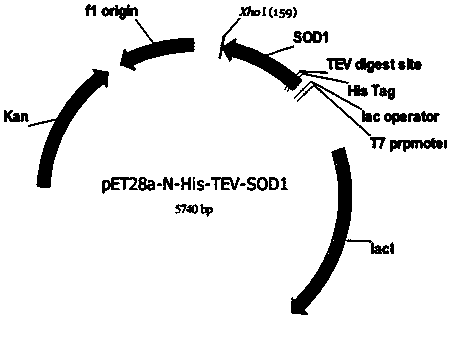
[0066] 2) Recombinant vector pET28a-N-His-TEV-hSOD1 transforms Escherichia coli competent cells
[0067] Mix 2 μL of the recombinant vector pET28a-N-His-TEV-hSOD1 (the content does not ex...
Embodiment 3
[0071] Embodiment 3: recombinant bacterial strain produces hSOD1
[0072] 1) The recombinant strain was induced by IPTG to produce hSOD1
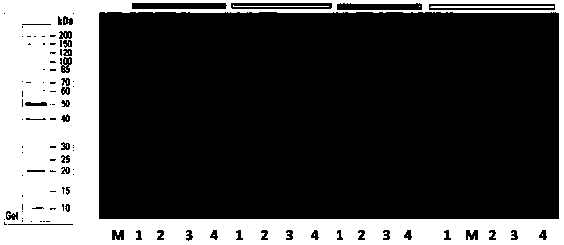
[0073] Take 20 μL of the bacterial solution in the glycerol preservation tube in Example 2 and inoculate it into 200 ml of LB liquid medium containing antibiotics, cultivate it at 37° C., 140 rpm to OD 600 Up to 0.4 ~ 0.6. Transfer to 1000ml LB liquid culture medium containing antibiotics according to 2.5% inoculum amount. When cultured with shaking at 37°C to OD 600 When the concentration reaches 0.4-1.0, add IPTG to a final concentration of 0.3 mM, and induce at 30° C. for 4 hours. Bacteria were collected by centrifugation. SDS-PAGE detection was carried out to analyze the expression and enzyme activity of hSOD1.
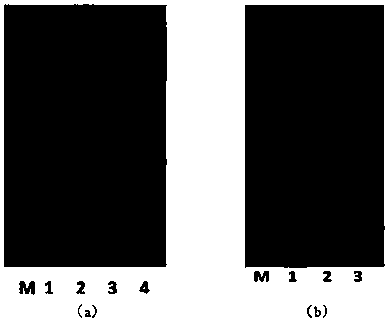
[0074] image 3Middle (a) is the production of hSOD1 induced by IPTG. As shown in the figure, M is the protein molecular weight standard; Lane 1 is the whole cell; Lane 2 is the broken whole cell; Lane 3 is the broken su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com