Chimeric antibacterial cell-permeable peptide T11N2 and application thereof
A T11N2, antimicrobial peptide technology, applied in the chimeric transmembrane antimicrobial peptide T11N2 and its application field, can solve the problems of restriction elimination, large cell stimulation, weak targeting, etc., and achieves low cost, relatively easy synthesis, and structural simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1 Preparation of chimeric membrane-penetrating antimicrobial peptide
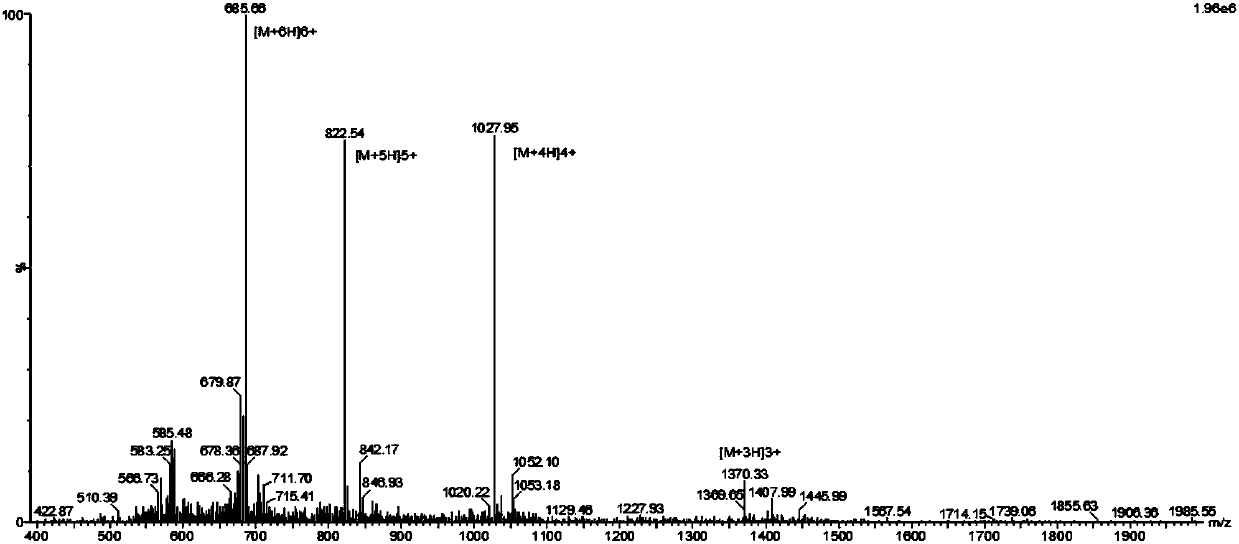
[0039] On the basis of highly active antimicrobial peptide N2 (SEQ ID NO:2), a cell-penetrating peptide Tat with 11 amino acids was connected to design a novel chimeric antimicrobial peptide T11N2, whose amino acid sequence is shown in SEQ ID NO:1 .
[0040] Antimicrobial peptide T11N2 can be prepared by Fmoc solid phase synthesis.
[0041] 1. Synthesis sequence: from the C-terminal to the N-terminal of the sequence, the steps are as follows:
[0042] 1) Weigh n equivalents of resin into the reactor, add DCM to swell for half an hour, then remove the DCM, add 2n equivalents of the first amino acid (Fmoc-Asn(Trt)-OH) in the sequence, add 2n equivalents of DIEA, Appropriate amount of DMF, DCM (appropriate amount means that the resin can fully expand), DIEA (diisopropylethylamine), DMF (N,N-dimethylformamide), DCM, blowing nitrogen for 60min, and then adding 5n equivalent methanol , reacte...
Embodiment 2
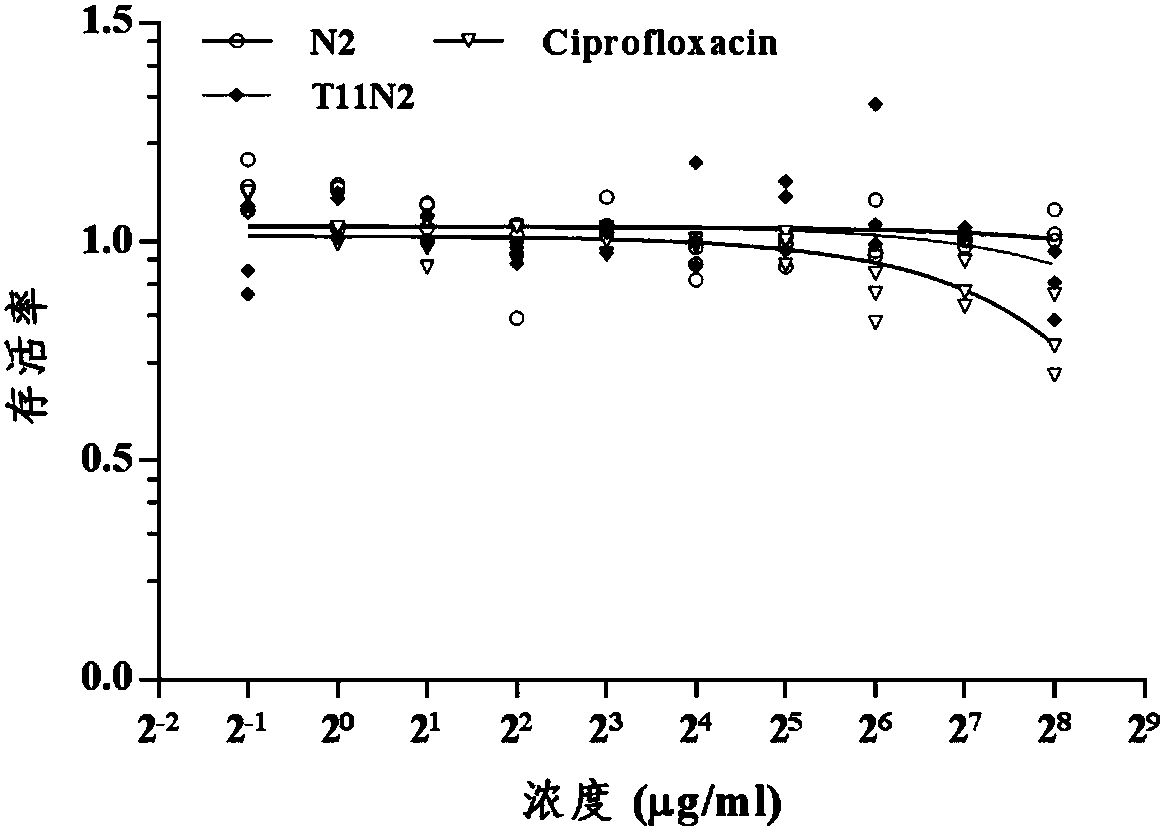
[0055] Example 2 Cytotoxicity Experiment of Chimeric Membrane-penetrating Antimicrobial Peptides
[0056] 1. Collect logarithmic phase RAW264.7 cells (mouse peritoneal macrophage cell line), adjust the concentration of cell suspension, add 7×10 3 cells, 5% CO 2 , and incubated at 37°C until the cell monolayer covered the bottom of the well (96-well flat bottom plate).
[0057] 2. Prepare different concentrations of antimicrobial peptide T11N2 solutions (dissolved in 0.01M phosphate buffer), and set up three negative control wells at the same time, 37 ° C, 5% CO 2 Cultivate for 1-5h.
[0058] 3. Add 20ul of MTT solution to each well, continue to cultivate for 4 hours, stop the culture, and carefully suck off the culture medium in the well.
[0059] 4. Add 150ul dimethyl sulfoxide to each well, shake on a shaker at low speed for 10min. The absorbance of each well was measured at OD490nm in an enzyme-linked immunosorbent assay instrument.
[0060] 5. At the same time, set th...
Embodiment 3
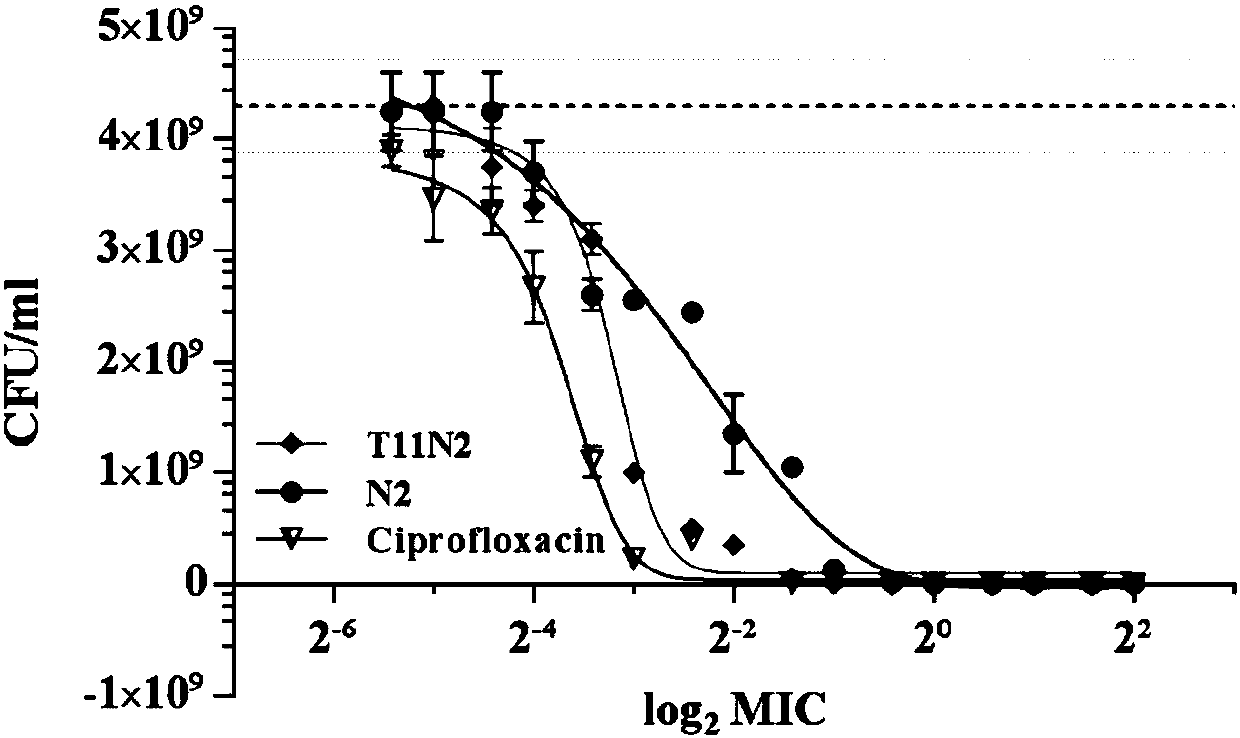
[0062] Example 3 Detection of Anti-Salmonella Activity
[0063] The minimum inhibitory concentration (MIC) of the antimicrobial peptide T11N2 against Salmonella was established with reference to Tian et al. Micro broth dilution method, slightly modified according to the specific situation, the specific operation is as follows:
[0064] 1) Pick a single clone of the tested strain to MH medium, culture at 37°C, 250rpm, shake overnight;
[0065] 2) Serially dilute the antibacterial drugs into 1.5mL sterile centrifuge tubes according to a 2-fold gradient, and the concentrations are 10 times the final concentrations;
[0066] 3) Transfer the tested strains to MH liquid medium at 37°C with 1% inoculum amount, and shake at 250rpm to 0.5 McFarland standard turbidity;
[0067] 4) Dilute 1000 times of the test bacteria culture solution (the final bacterium concentration is about 10 5 CFU / mL), transferred to a sterile cell culture plate, each well containing 90 μL of diluted bacterial...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com