Solar nitrogen fixation pholocatalyst as well as purpose and preparation method thereof
A photocatalyst and solar energy technology, applied in the field of photocatalytic materials, can solve problems such as weak binding ability, and achieve the effects of effective nitrogen fixation performance, broad development space, and great application potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Heat 40ml of ethylene glycol solution to 160°C and cool down to 120°C. 1.92g of bismuth nitrate pentahydrate and 0.416g of sodium bromide were added to the above solution, mixed and fully stirred for 35 minutes to a clear solution, then 120ml of isopropanol solution was added, the resulting solution was stirred for 30 minutes, then put into a 1-2Mpa high-pressure reactor, and kept at a constant temperature of 160°C Hydrothermal reaction 12h. The product was cooled to room temperature, centrifuged and washed 5 times with water and ethanol solution, dried at 60°C and ground to powder. Calcined at 200°C and 360ml / min hydrogen atmosphere for 4h to obtain the final product H-BiOBr.
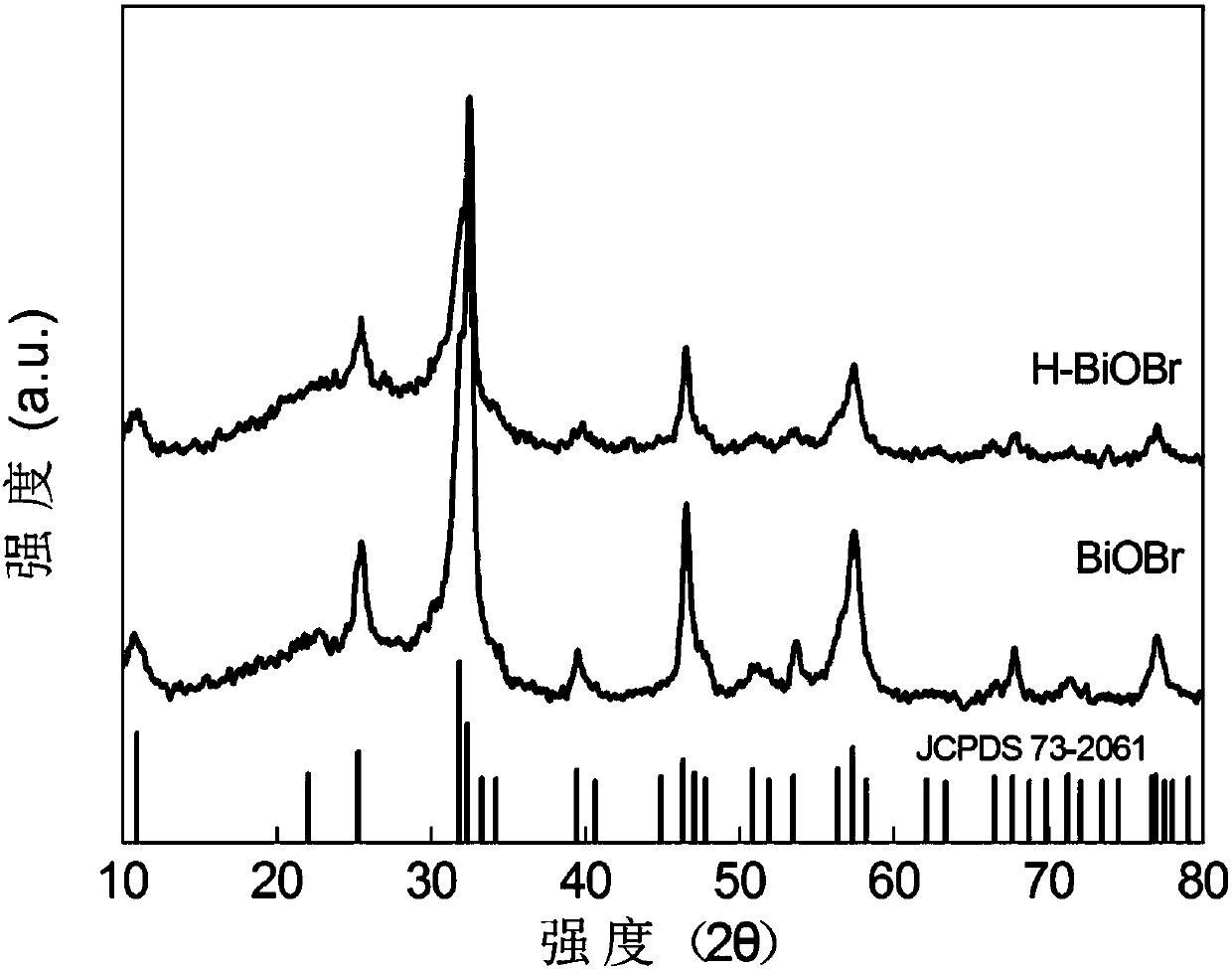
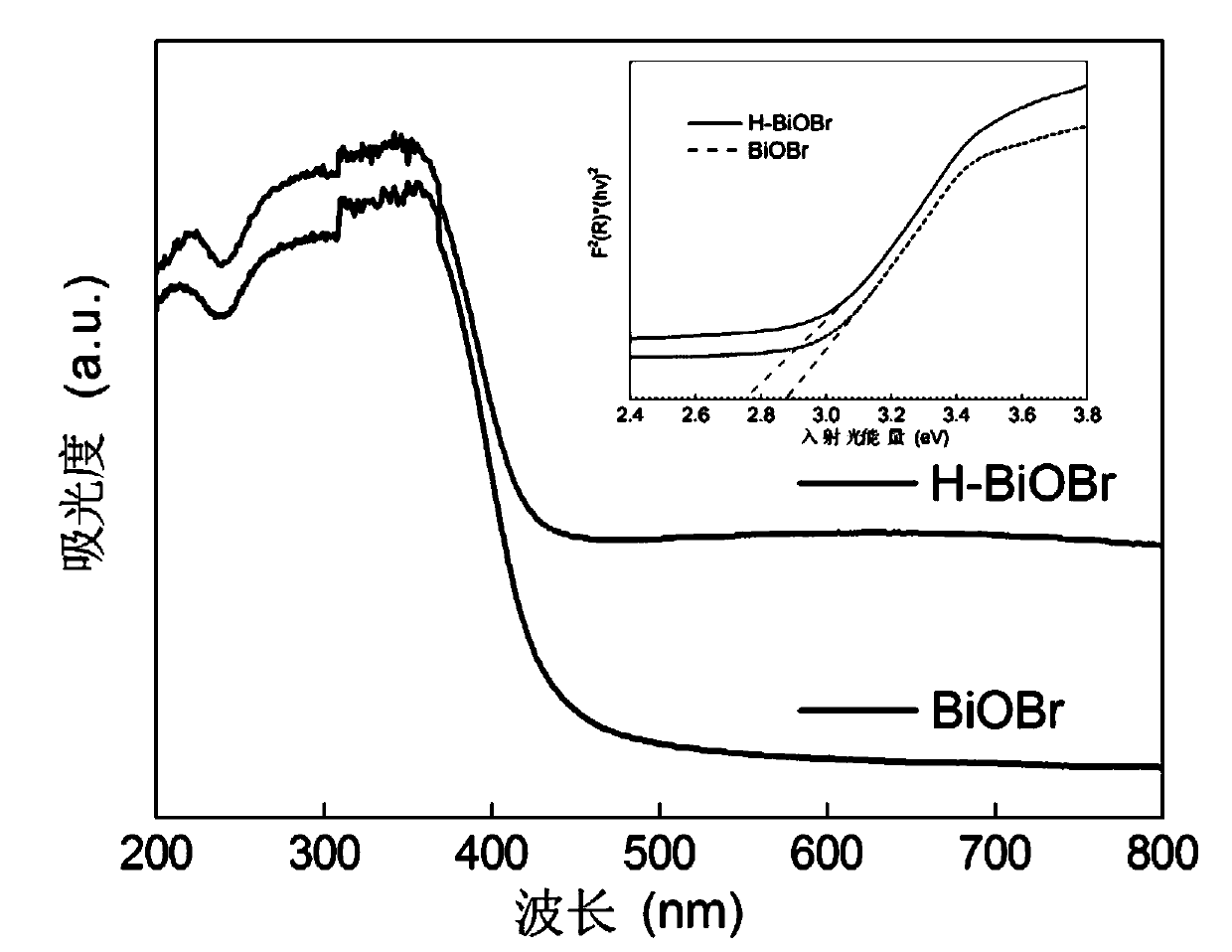
[0042] figure 1 It is the XRD diffraction pattern of the H-BiOBr photocatalyst obtained in this example, and compared with the PDF standard card, it is known that the obtained BiOBr is a tetragonal phase.
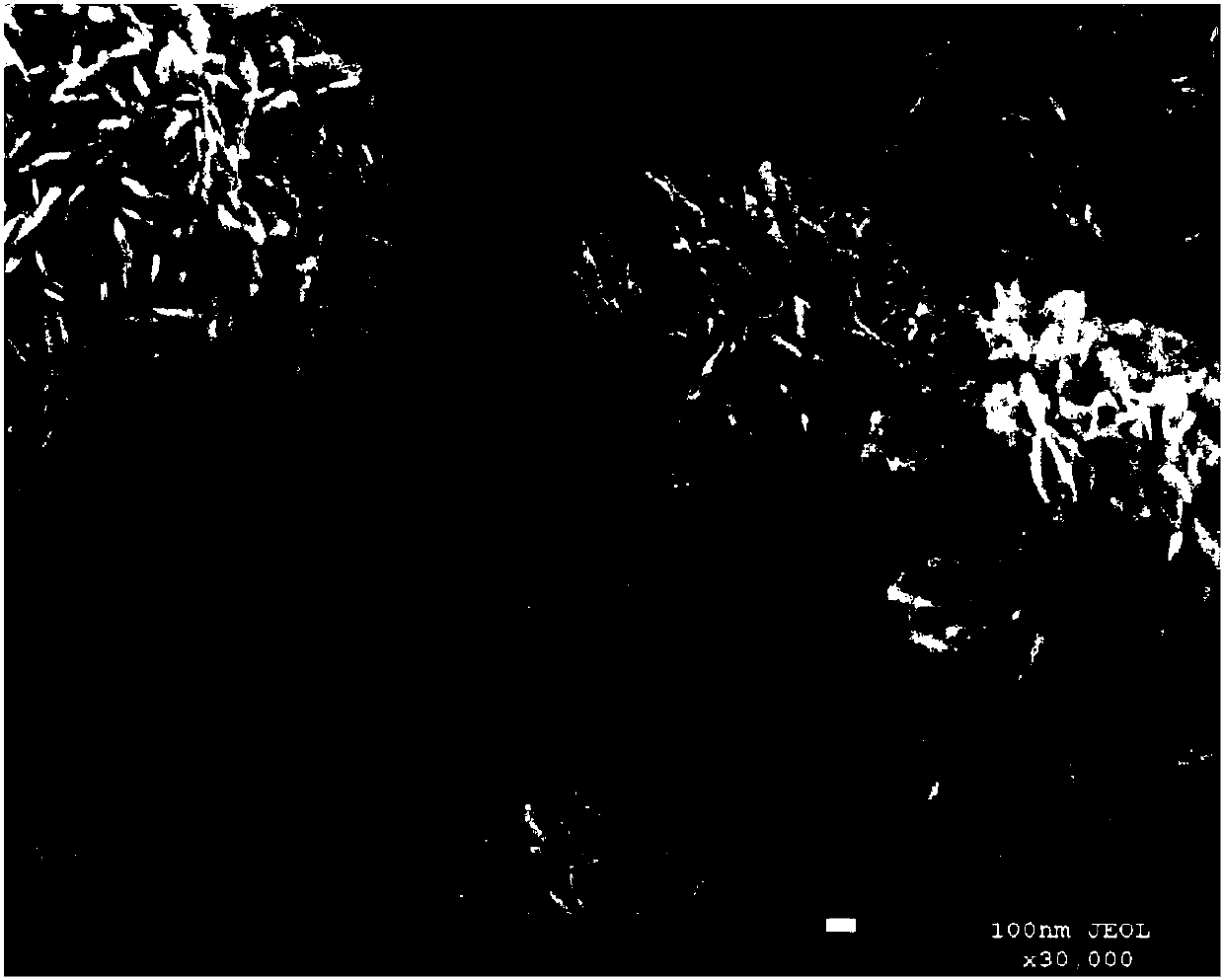
[0043] figure 2 It is the scanning electron microscope picture of the H-BiOBr photoc...
Embodiment 2
[0048] Heat 20ml of ethylene glycol solution to 200°C and cool down to 60°C. Add 0.24g of bismuth nitrate pentahydrate and 0.052g of sodium bromide to the above solution, mix and stir thoroughly for 30 minutes until the solution becomes clear, then add 60ml of isopropanol solution, stir the resulting solution for 30 minutes, put it into a high-pressure reactor, and perform a constant temperature hydrothermal reaction at 160°C 20h. The product was cooled to room temperature, centrifuged and washed 5 times with water and ethanol solution, dried at 60°C and ground to powder. Calcined at 150°C and 360ml / min hydrogen atmosphere for 4h to obtain the final product H-BiOBr.
Embodiment 3
[0050] Heat 20ml of ethylene glycol solution to 200°C and cool down to 60°C. Add 0.24g of bismuth nitrate pentahydrate and 0.052g of sodium bromide to the above solution, mix and stir thoroughly for 30 minutes until the solution becomes clear, then add 60ml of isopropanol solution, stir the resulting solution for 30 minutes, put it into a high-pressure reactor, and perform a constant temperature hydrothermal reaction at 160°C 20h. The product was cooled to room temperature, centrifuged and washed 5 times with water and ethanol solution, dried at 60°C and ground to powder. Calcined at 150°C and 360ml / min hydrogen atmosphere for 2h to obtain the final product H-BiOBr.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com