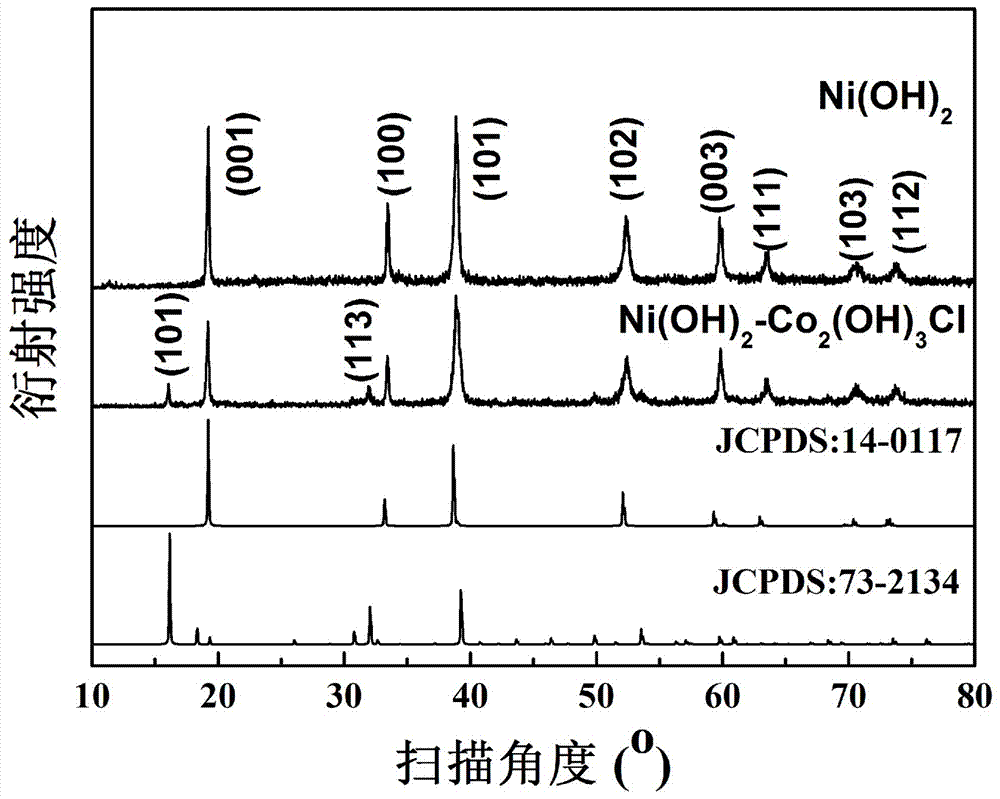
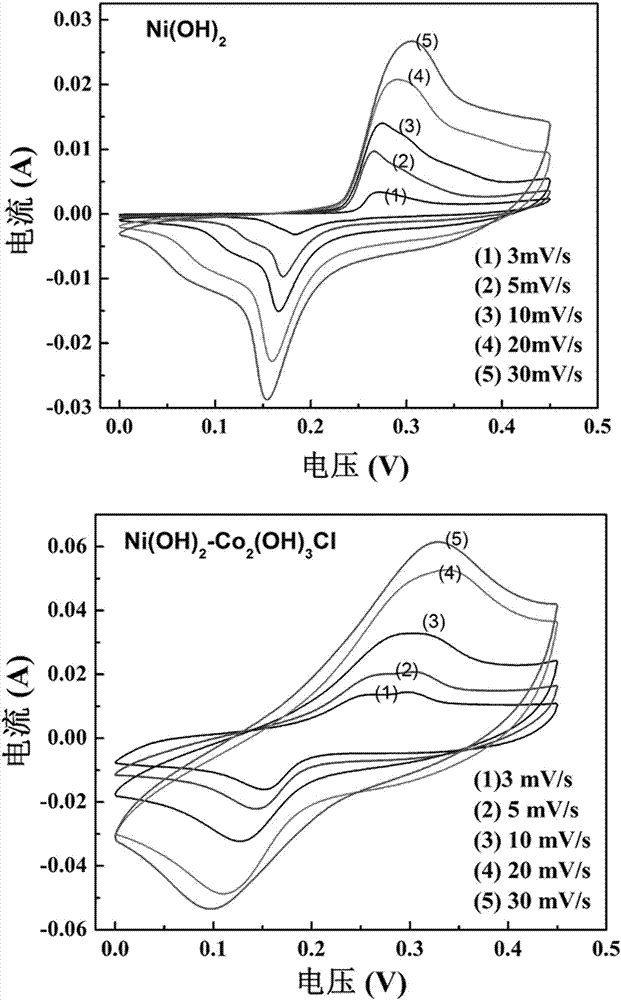
Ni(OH)2-Co2(OH)3Cl composite nanometer array electrode materials
A nano-array, electrode material technology, applied in hybrid capacitor electrodes, nanotechnology, nanotechnology and other directions, can solve the problems of unsatisfactory electrochemical performance, reduced performance, single electrochemical reaction, etc., to achieve low cost, simple operation, raw materials cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] ①The commercially available thickness is 1.5mm, surface density is 280-420g / m 2The nickel foam with a pore size of 0.2-0.6mm is cut into a size of 1cm×4cm, and is ultrasonically treated with 3mol / L hydrochloric acid for 30 minutes to remove surface oxides. Wash with ethanol and deionized water alternately, and dry in vacuum for later use.
[0028] ②In a beaker, add 1.6mmol of nickel chloride, 1.6mmol of urea, and 1.6mmol of ammonium fluoride into 16mL of water, stir and sonicate for 15 minutes until completely dissolved. Move the solution into a 25mL polytetrafluoroethylene liner high-pressure reactor, and then put the pretreated 1cm×4cm nickel foam into the reactor, react at 100°C for 12 hours, cool to room temperature naturally, and wash with deionized water and ethanol Wash several times and dry the sample in vacuum to obtain Ni(OH) grown on nickel foam 2 nanoarray.
[0029] ③In a beaker, add 1.6mmol of cobalt chloride to 16mL of water, and stir until completely d...
Embodiment 2
[0034] ①The commercially available thickness is 1.5mm, surface density is 280-420g / m 2 The nickel foam with a pore size of 0.2-0.6mm is cut into a size of 1cm×4cm, and is ultrasonically treated with 3mol / L hydrochloric acid for 30 minutes to remove surface oxides. Wash with ethanol and deionized water alternately, and dry in vacuum for later use.
[0035] ②In a beaker, add 0.8mmol of nickel sulfate, 0.8mmol of urea, and 0.8mmol of ammonium fluoride into 16mL of water, and stir until completely dissolved. Move the solution into a 25mL polytetrafluoroethylene liner high-pressure reactor, then put the pretreated 1cm×4cm foam nickel into the reactor, react at 120°C for 12 hours, cool to room temperature naturally, and wash with deionized water and ethanol Several times, dry the sample in vacuum to obtain Ni(OH) grown on nickel foam 2 nanoarray.
[0036] ③In a beaker, add 0.8mmol of cobalt chloride to 16mL of water and stir until completely dissolved. The solution is moved into...
Embodiment 3
[0039] ①The commercially available thickness is 1.5mm, surface density is 280-420g / m 2 The nickel foam with a pore size of 0.2-0.6mm is cut into a size of 1cm×4cm, and is ultrasonically treated with 3mol / L hydrochloric acid for 30 minutes to remove surface oxides. Wash with ethanol and deionized water alternately, and dry in vacuum for later use.
[0040] ②In a beaker, add 3.2mmol of nickel acetate, 3.2mmol of urea, and 3.2mmol of ammonium fluoride into 16mL of water, and stir until completely dissolved. Move the solution into a 25mL polytetrafluoroethylene liner high-pressure reactor, then put the pretreated 1cm×4cm foam nickel into the reactor, react at 100°C for 24 hours, cool to room temperature naturally, and wash with deionized water and ethanol Several times, dry the sample in vacuum to obtain Ni(OH) grown on nickel foam 2 nanoarray.
[0041] ③In a beaker, add 3.2mmol of cobalt chloride to 16mL of water and stir until completely dissolved. The solution is moved into...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Areal density | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com