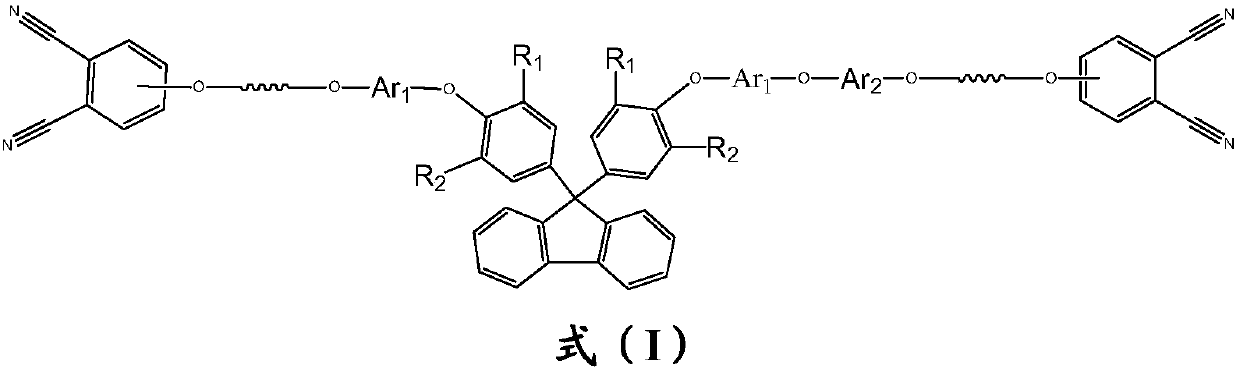
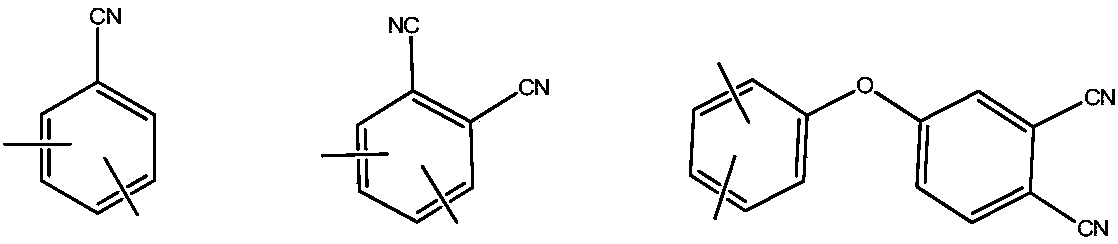
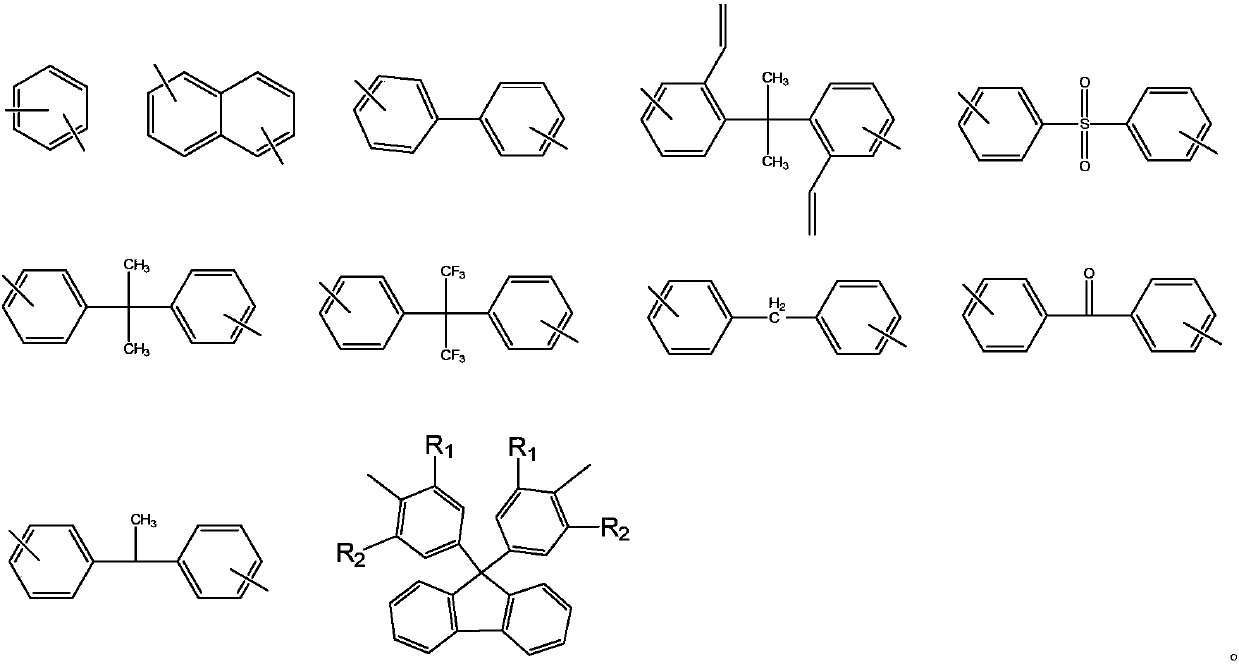
Phthalonitrile-terminated fluorine structure-containing poly(arylene ether nitrile) oligomer, phthalonitrile-terminated fluorine structure-containing poly(arylene ether nitrile)condensate, and preparation method of oligomer
A technology of phthalonitrile and poly(arylene ether nitrile), which is applied to cured products and their preparation, and the field of phthalonitrile-terminated poly(arylene ether nitrile) oligomers containing fluorene structure, which can solve the problem of unmentioned oligomers. , cured product and its preparation method, etc., to achieve the effect of good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] 1) Add 8g (0.0465mol) of dichlorobenzonitrile, 32.59g (0.093mol) of bisphenol fluorene, 25.71g (0.186mol) of anhydrous potassium carbonate, 80mL of NMP and 20mL of toluene into the three-port port with water separation and condensation reflux device In the flask, under the protection of nitrogen, heat to 130-145°C to divide water for 4 hours, slowly distill out toluene after the completion of water separation, control the reaction temperature by distilling the speed and amount of toluene, continue to react at 150-200°C for 4 hours, cool to 80 Below ℃;
[0074] 2) Add 16.9 g (0.098 mol) of 4-nitrophthalonitrile to the reaction liquid in step 1), and react at a temperature of 80° C. for 12 hours;
[0075] 3) Filter the reaction solution described in step 2), remove the catalyst, recover the solvent under reduced pressure at 40-80°C in the filtrate, pour the residue into 5wt% hydrochloric acid solution for sedimentation, filter, and stir and wash with deionized water for 2...
Embodiment 2
[0085] 1) Add 8g (0.0465mol) of dichlorobenzonitrile, 21.73g (0.062mol) of bisphenol fluorene, 17.14g (0.128mol) of anhydrous potassium carbonate, 80mL of NMP and 20mL of toluene into the three-port port with water separation and condensation reflux device In the flask, under the protection of nitrogen, heat to 130-145°C to separate the water for 3 hours, slowly distill out the toluene after the completion of the water separation, control the reaction temperature by distilling the speed and amount of toluene to continue the reaction at 150-200°C for 5 hours, cool to 80 Below ℃;
[0086] 2) Add 5.90 g (0.065 mol) of 4-nitrophthalonitrile to the reaction solution in step 1), and react at a temperature of 80° C. for 12 hours.
[0087] 3) Filter the reaction solution described in step 2), remove the catalyst, recover the solvent under reduced pressure at 40-80° C. from the filtrate, pour the residue into 8 wt% hydrochloric acid solution for sedimentation, filter, and stir and wash...
Embodiment 3
[0092] 1) Add 8g (0.0465mol) of dichlorobenzonitrile, 20.37g (0.058mol) of bisphenol fluorene, 16.06g (0.116mol) of anhydrous potassium carbonate, 80mL of NMP and 20mL of toluene into the three-port port with water separation and condensation reflux device In the flask, under the protection of nitrogen, heat to 130-145°C to divide water for 2.5 hours, slowly distill out toluene after water separation, control the reaction temperature by distilling the speed and amount of toluene at 150-200°C for 6 hours, cool to 80 Below ℃;
[0093] 2) Add 4.43 g (0.061 mol) of 4-nitrophthalonitrile to the reaction solution described in step 1), and react at a temperature of 85° C. for 12 hours.
[0094] 3) Filter the reaction solution described in step 2), remove the catalyst, recover the solvent under reduced pressure at 40-80° C. from the filtrate, pour the residue into 8 wt% hydrochloric acid solution for sedimentation, filter, and stir and wash with deionized water 2-3 times until The fi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| bending strength | aaaaa | aaaaa |
| bending strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com