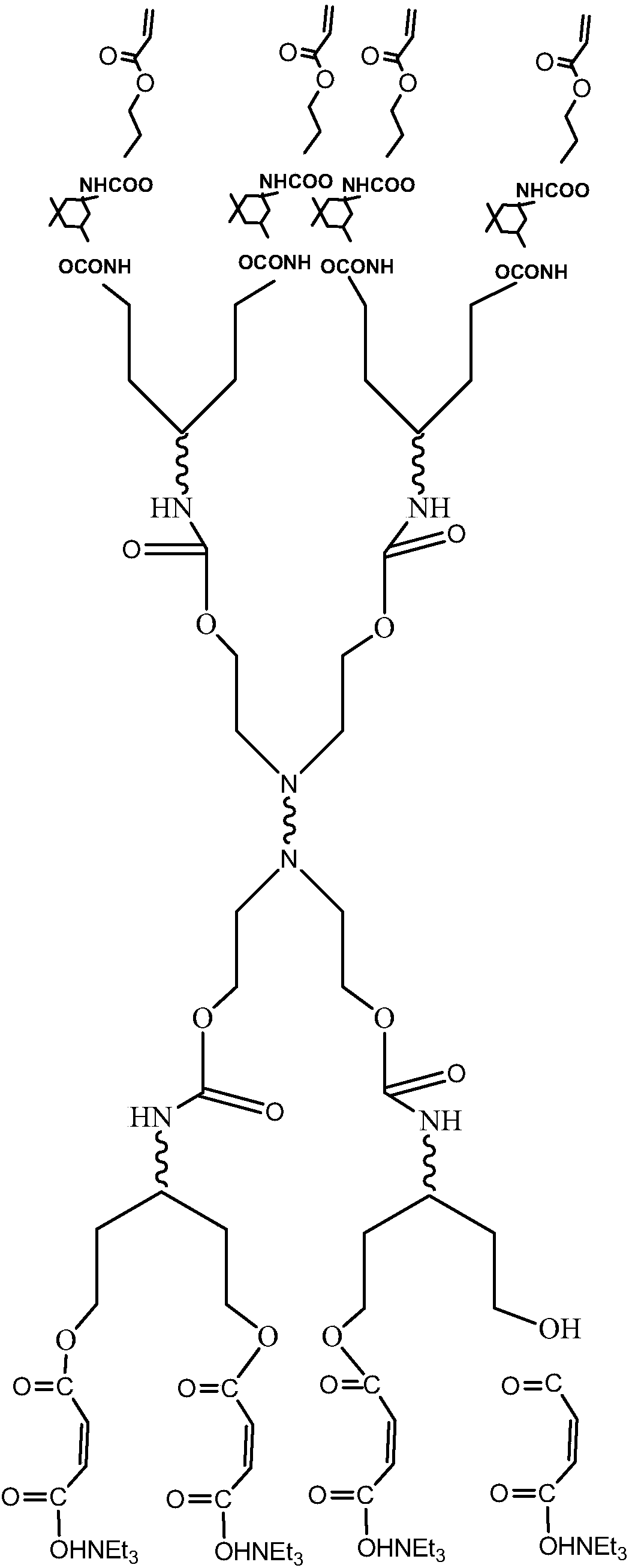
UV curing water-based hyperbranched polyurethane acrylic ester and preparation method thereof
A polyurethane acrylate and acrylate technology, which is applied in the field of polymer materials, can solve the problems of poor resin flexibility, high acrylate hard segment content, and high hard segment content, and achieve industrial production convenience, broad application prospects, and wide sources of raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
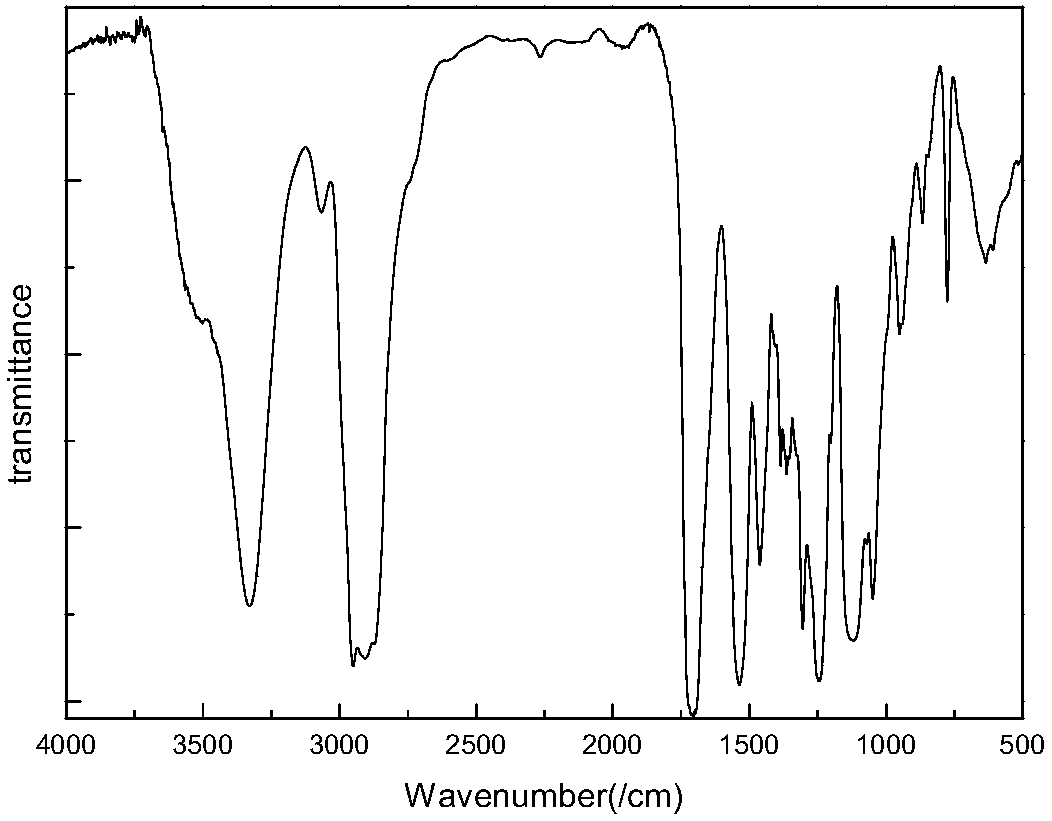
Image
Examples
Embodiment 1
[0026] 2 g of polyethylene glycol-200 (PEG-200) (0.01 mol) and 4.44 g of isophorone diisocyanate (IPDI) (0.02 mol) were dissolved in 8.54 g of N,N-dimethylformamide ( DMF), and add 0.02g catalyst tetraisopropyl titanate, feed nitrogen, and react for 3 hours to obtain a colorless transparent liquid; lower the temperature of the system to 0°C, add 2.1g diethanolamine (0.02mol) dropwise, drop Adding time is 30min, reaction is 2 hours.
[0027] 8.89g of isophorone diisocyanate (IPDI) (0.04mol) and 4.2g of diethanolamine (0.04mol) were added to 13.08g of N,N-dimethylformamide (DMF) under stirring at 0°C for 2 hours .
[0028] The products obtained after the above two steps of reaction were mixed, and reacted for 4 hours under stirring at 40° C. to obtain hyperbranched polyurethane.
[0029] 8.89g of isophorone diisocyanate (IPDI) (0.04mol) was added dropwise to 4.65g of hydroxyethyl acrylate (HEA) (0.04mol) and 13.52g of N,N-dimethylformamide (DMF) under stirring at 40°C ) in th...
Embodiment 2
[0031]1.34 g of 2,2-dihydroxymethylpropionic acid (DMPA) (0.01 mol) and 4.44 g of isophorone diisocyanate (IPDI) (0.02 mol) were dissolved in 5.78 g of N,N-dimethyl Formamide (DMF), and add 0.02g catalyst dibutyltin dilaurate, feed nitrogen, react for 4 hours to obtain a colorless transparent liquid; drop the temperature of the system to 0 ° C, dropwise add 2.1g diethanolamine (0.02mol) , the dropping time is 30min, and the reaction is 2 hours.
[0032] Add 17.78g of isophorone diisocyanate (IPDI) (0.08mol) and 8.41g of diethanolamine (0.08mol) to 13.08g of N,N-dimethylformamide (DMF) under stirring at 0°C, and blow nitrogen , reacted for 2 hours.
[0033] Mix the products obtained after the above two steps of reaction, raise the temperature to 40° C., stir and react for 5 hours, and obtain hyperbranched polyurethane.
[0034] Add 17.78g of isophorone diisocyanate (IPDI) (0.08mol) dropwise to 9.44g of hydroxyethyl methacrylate (0.08mol) and 13.62g of N,N-dimethylformamide (D...
Embodiment 3
[0036] 0.901 g of 1,4-butanediol (BDO) (0.01 mol) and 4.44 g of isophorone diisocyanate (IPDI) (0.02 mol) were dissolved in 5.43 g of N,N-dimethylformamide (DMF) under stirring at 30°C. ), and add 0.02g catalyst dibutyltin dilaurate, feed nitrogen, and react for 3 hours to obtain a colorless transparent liquid; the temperature of the system is lowered to 0°C, and 2.1g diethanolamine (0.02mol) is added dropwise. 30min, 4 hours of reaction.
[0037] Add 8.89g of isophorone diisocyanate (IPDI) (0.04mol) and 4.2g of diethanolamine (0.04mol) to 13.08g of N,N-dimethylformamide (DMF) under stirring at 5°C, and blow nitrogen , reacted for 2 hours.
[0038] Mix the products obtained after the above two steps of reaction, raise the temperature to 40° C. and react under stirring for 6 hours to obtain hyperbranched polyurethane.
[0039] 8.89g of isophorone diisocyanate (IPDI) (0.04mol) was added dropwise to 4.65g of hydroxyethyl acrylate (HEA) (0.04mol) and 13.55g of N,N-dimethylformam...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com